Changes to aspects of ongoing randomised controlled trials with fixed designs
- PMID: 32493444
- PMCID: PMC7268339
- DOI: 10.1186/s13063-020-04374-3
Changes to aspects of ongoing randomised controlled trials with fixed designs
Abstract
Background: Despite careful planning, changes to some aspects of an ongoing randomised clinical trial (RCT), with a fixed design, may be warranted. We sought to elucidate the distinction between legitimate versus illegitimate changes to serve as a guide for less experienced clinical trialists and other stakeholders.
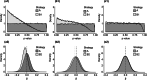
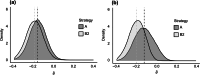
Methods: Using data from a large trial of statin therapy for secondary prevention, we generated a set of simulated trial datasets under the null hypothesis (H0) and a set under an alternative hypothesis (H1). Through analysis of these simulated trials, we assessed the performance of the strategy of changing aspects of the design/analysis with knowledge of treatment allocation (illegitimate) versus the strategy of making changes without knowledge of treatment allocation (legitimate). Performance was assessed using the type 1 error, as well as measures of absolute and relative bias in the treatment effect.
Results: Illegitimate changes led to a relative bias of 61% under H1, and a type 1 error rate under H0 of 23%-well in excess of the 5% significance level targeted. Legitimate changes produced unbiased estimates under H1 and did not inflate the type 1 error rate under H0.
Conclusions: Changes to pre-specified aspects of the design and analysis of an ongoing RCT may be a necessary response to unforeseen circumstances. Such changes risk introducing a bias if undertaken with knowledge of treatment allocation. Legitimate changes need to be adequately documented to provide assurance to all stakeholders of their validity.
Keywords: Bias; Design changes; Randomised control trials; Type 1 error.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- FIELD Study Investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–61. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources