Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis
- PMID: 32493740
- PMCID: PMC7828899
- DOI: 10.1503/cmaj.200647
Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis
Abstract
Background: Antiviral medications are being given empirically to some patients with coronavirus disease 2019 (COVID-19). To support the development of a COVID-19 management guideline, we conducted a systematic review that addressed the benefits and harms of 7 antiviral treatments for COVID-19.
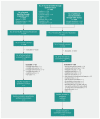
Methods: We searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PubMed and 3 Chinese databases (CNKI, WANFANG and SinoMed) through Apr. 19, medRxiv and Chinaxiv through Apr. 27, and Chongqing VIP through Apr. 30, 2020. We included studies of ribavirin, chloroquine, hydroxychloroquine, umifenovir (arbidol), favipravir, interferon and lopinavir/ritonavir. If direct evidence from COVID-19 studies was not available, we included indirect evidence from studies of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) for efficacy outcomes and other acute respiratory viral infections for safety outcomes.
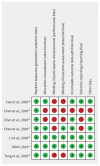
Results: In patients with nonsevere COVID-19 illness, the death rate was extremely low, precluding an important effect on mortality. We found only very low-quality evidence with little or no suggestion of benefit for most treatments and outcomes in both nonsevere and severe COVID-19. An exception was treatment with lopinavir/ritonavir, for which we found low-quality evidence for a decrease in length of stay in the intensive care unit (risk difference 5 d shorter, 95% confidence interval [CI] 0 to 9 d) and hospital stay (risk difference 1 d shorter, 95% CI 0 to 2 d). For safety outcomes, evidence was of low or very low quality, with the exception of treatment with lopinavir/ritonavir for which moderate-quality evidence suggested likely increases in diarrhea, nausea and vomiting.
Interpretation: To date, persuasive evidence of important benefit in COVID-19 does not exist for any antiviral treatments, although for each treatment evidence has not excluded important benefit. Additional randomized controlled trials involving patients with COVID-19 will be needed before such treatments can be administered with confidence.
© 2020 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Ning Shen is an investigator in a clinical trial evaluating the effect of hydroxychloroquine in patients with coronavirus disease 2019 that is funded by the Peking University Health Science Center. No other competing interests were declared.
Figures
Comment in
- CMAJ. 192:E536.
References
-
- Novel coronavirus (2019-nCoV) situation report — 22. Geneva: World Health Organization; 2020. Available: www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-... (accessed 2020 Mar. 3).
-
- Coronavirus disease 2019 (COVID-19) situation report — 101. Geneva: World Health Organization; 2020. Available: www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-... (accessed 2020 May 1).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous