Streptococcus pyogenes genes that promote pharyngitis in primates
- PMID: 32493846
- PMCID: PMC7308061
- DOI: 10.1172/jci.insight.137686
Streptococcus pyogenes genes that promote pharyngitis in primates
Abstract
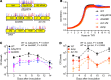
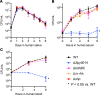
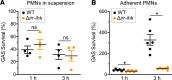
Streptococcus pyogenes (group A streptococcus; GAS) causes 600 million cases of pharyngitis annually worldwide. There is no licensed human GAS vaccine despite a century of research. Although the human oropharynx is the primary site of GAS infection, the pathogenic genes and molecular processes used to colonize, cause disease, and persist in the upper respiratory tract are poorly understood. Using dense transposon mutant libraries made with serotype M1 and M28 GAS strains and transposon-directed insertion sequencing, we performed genome-wide screens in the nonhuman primate (NHP) oropharynx. We identified many potentially novel GAS fitness genes, including a common set of 115 genes that contribute to fitness in both genetically distinct GAS strains during experimental NHP pharyngitis. Targeted deletion of 4 identified fitness genes/operons confirmed that our newly identified targets are critical for GAS virulence during experimental pharyngitis. Our screens discovered many surface-exposed or secreted proteins - substrates for vaccine research - that potentially contribute to GAS pharyngitis, including lipoprotein HitA. Pooled human immune globulin reacted with purified HitA, suggesting that humans produce antibodies against this lipoprotein. Our findings provide new information about GAS fitness in the upper respiratory tract that may assist in translational research, including developing novel vaccines.
Keywords: Bacterial infections; Infectious disease.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

