Hair-bearing human skin generated entirely from pluripotent stem cells
- PMID: 32494013
- PMCID: PMC7593871
- DOI: 10.1038/s41586-020-2352-3
Hair-bearing human skin generated entirely from pluripotent stem cells
Abstract
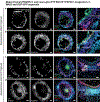
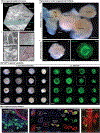
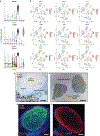
The skin is a multilayered organ, equipped with appendages (that is, follicles and glands), that is critical for regulating body temperature and the retention of bodily fluids, guarding against external stresses and mediating the sensation of touch and pain1,2. Reconstructing appendage-bearing skin in cultures and in bioengineered grafts is a biomedical challenge that has yet to be met3-9. Here we report an organoid culture system that generates complex skin from human pluripotent stem cells. We use stepwise modulation of the transforming growth factor β (TGFβ) and fibroblast growth factor (FGF) signalling pathways to co-induce cranial epithelial cells and neural crest cells within a spherical cell aggregate. During an incubation period of 4-5 months, we observe the emergence of a cyst-like skin organoid composed of stratified epidermis, fat-rich dermis and pigmented hair follicles that are equipped with sebaceous glands. A network of sensory neurons and Schwann cells form nerve-like bundles that target Merkel cells in organoid hair follicles, mimicking the neural circuitry associated with human touch. Single-cell RNA sequencing and direct comparison to fetal specimens suggest that the skin organoids are equivalent to the facial skin of human fetuses in the second trimester of development. Moreover, we show that skin organoids form planar hair-bearing skin when grafted onto nude mice. Together, our results demonstrate that nearly complete skin can self-assemble in vitro and be used to reconstitute skin in vivo. We anticipate that our skin organoids will provide a foundation for future studies of human skin development, disease modelling and reconstructive surgery.
Conflict of interest statement
COMPETING INTERESTS
J.L. and K.R.K, with the Indiana University Research and Technology Corporation, have submitted a patent application covering the entire skin organoid induction method and including much of the data presented in this manuscript (WO2017070506A1). The other authors declare no competing interests.
Figures








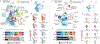


Comment in
-
Regenerative medicine could pave the way to treating baldness.Nature. 2020 Jun;582(7812):343-344. doi: 10.1038/d41586-020-01568-2. Nature. 2020. PMID: 32541911 No abstract available.
References
-
- Sun BK, Siprashvili Z & Khavari PA Advances in skin grafting and treatment of cutaneous wounds. Science 346, 941–945 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

