Efficacy and Safety of Evocalcet Evaluated by Dialysate Calcium Concentration in Patients with Secondary Hyperparathyroidism Undergoing Hemodialysis
- PMID: 32494184
- PMCID: PMC7229806
- DOI: 10.2147/IJNRD.S243210
Efficacy and Safety of Evocalcet Evaluated by Dialysate Calcium Concentration in Patients with Secondary Hyperparathyroidism Undergoing Hemodialysis
Abstract
Purpose: Evocalcet is a novel oral calcimimetic drug that has demonstrated similar efficacy to cinacalcet in regulating serum parathyroid hormone (PTH), calcium, and phosphate levels, with fewer upper gastrointestinal tract-related adverse drug reactions (ADRs) in patients with secondary hyperparathyroidism undergoing hemodialysis in Japan. We investigated the efficacy and safety of once-daily oral evocalcet under different dialysate calcium concentrations.
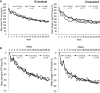
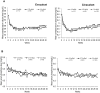
Patients and methods: A post hoc analysis by dialysate calcium concentration (2.5, 2.75, and 3.0 mEq/L) was performed using data from a previous Phase 3 study that included cinacalcet as an active control. Efficacy endpoints were the proportion of patients who achieved the target intact PTH levels of ≥60 and ≤240 pg/mL between Week 28 and Week 30; time-course changes in serum intact PTH; calcium and phosphorus levels, bone turnover markers, and fibroblast growth factor 23 (FGF23) over the 30-week study period. Safety endpoints were overall ADRs and hypocalcemia- and upper gastrointestinal tract-related ADRs.
Results: A total of 634 patients were included in the analysis. Levels of intact PTH, calcium, phosphate, bone turnover markers, and FGF23 showed improvement in all sub-groups, irrespective of dialysate calcium concentration. The incidence of upper gastrointestinal tract-related ADRs was significantly lower in the evocalcet group than the cinacalcet group with dialysate calcium concentrations of 2.75 and 3.0 mEq/L (p<0.05 for both concentrations).
Conclusion: Evocalcet was effective and safe in regulating the levels of serum intact PTH, calcium, and phosphate in patients with secondary hyperparathyroidism undergoing hemodialysis, irrespective of dialysate calcium concentration.
Keywords: hypocalcemia; intact parathyroid hormone; oral calcimimetic; post hoc analysis; upper gastrointestinal tract.
© 2020 Shigematsu et al.
Conflict of interest statement
TS received consulting fees from KKC, Ono Pharmaceutical, Taisho Toyama Pharmaceutical, Fuji Pharma, and FUSO pharmaceutical industries, and lecture fees from KKC, Chugai Pharmaceutical, Bayer, Kissei Pharmaceutical, Torii Pharmaceutical, Ono Pharmaceutical, and FUSO pharmaceutical industries. SA, YE, and TK are employees of KKC. MF received consulting fees from KKC and Ono Pharmaceutical; lecture fees from KKC, Bayer, Torii Pharmaceutical, and Ono Pharmaceutical; and grants from KKC and Bayer. TA received consulting fees from KKC, Astellas Pharma, Bayer, Fuso Pharmaceutical, Japan Tobacco, Ono Pharmaceutical, Sanwa Chemical, Otsuka, GSK, and NIPRO, and lecture fees from KKC, Chugai Pharmaceutical, Bayer, Kissei Pharmaceutical, Torii Pharmaceutical, and Ono Pharmaceutical. The authors report no other conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources

