Fast transformation of 2D nanofiber membranes into pre-molded 3D scaffolds with biomimetic and oriented porous structure for biomedical applications
- PMID: 32494338
- PMCID: PMC7233601
- DOI: 10.1063/1.5144808
Fast transformation of 2D nanofiber membranes into pre-molded 3D scaffolds with biomimetic and oriented porous structure for biomedical applications
Abstract
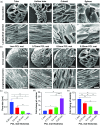
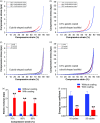
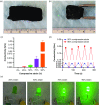
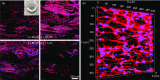
The ability to transform two-dimensional (2D) structures into three-dimensional (3D) structures leads to a variety of applications in fields such as soft electronics, soft robotics, and other biomedical-related fields. Previous reports have focused on using electrospun nanofibers due to their ability to mimic the extracellular matrix. These studies often lead to poor results due to the dense structures and small poor sizes of 2D nanofiber membranes. Using a unique method of combining innovative gas-foaming and molding technologies, we report the rapid transformation of 2D nanofiber membranes into predesigned 3D scaffolds with biomimetic and oriented porous structure. By adding a surfactant (pluronic F-127) to poly(ε-caprolactone) (PCL) nanofibers, the rate of expansion is dramatically enhanced due to the increase in hydrophilicity and subsequent gas bubble stability. Using this novel method together with molding, 3D objects with cylindrical, hollow cylindrical, cuboid, spherical, and irregular shapes are created. Interestingly, these 3D shapes exhibit anisotropy and consistent pore sizes throughout entire object. Through further treatment with gelatin, the scaffolds become superelastic and shape-recoverable. Additionally, gelatin-coated, cube-shaped scaffolds were further functionalized with polypyrrole coatings and exhibited dynamic electrical conductivity during cyclic compression. Cuboid-shaped scaffolds have been demonstrated to be effective for compressible hemorrhage in a porcine liver injury model. In addition, human neural progenitor cells can be uniformly distributed and differentiated into neurons throughout the cylinder-shaped nanofiber scaffolds, forming ordered 3D neural tissue constructs. Taken together, the approach presented in this study is very promising in the production of pre-molded 3D nanofiber scaffolds for many biomedical applications.
Figures



References
-
- Xu S., Yan Z., Jang K. I., Huang W., Fu H., Kim J., Wei Z., Flavin M., McCracken J., Wang R., Badea A., Liu Y., Xiao D., Zhou G., Lee J., Chung H. U., Cheng H., Ren W., Banks A., Li X., Paik U., Nuzzo R. G., Huang Y., Zhang Y., and Rogers J. A., Science 347(6218), 154 (2015).10.1126/science.1260960 - DOI - PubMed
-
- Fu H., Nan K., Bai W., Huang W., Bai K., Lu L., Zhou C., Liu Y., Liu F., Wang J., Han M., Yan Z., Luan H., Zhang Y., Zhang Y., Zhao J., Cheng X., Li M., Lee J. W., Liu Y., Fang D., Li X., Huang Y., Zhang Y., and Rogers J. A., Nat. Mater 17(3), 268 (2018).10.1038/s41563-017-0011-3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
