Left Atrial Appendage Occlusion: What Are the Options and Where is the Evidence?
- PMID: 32494488
- PMCID: PMC7252870
- DOI: 10.19102/icrm.2018.090402
Left Atrial Appendage Occlusion: What Are the Options and Where is the Evidence?
Abstract
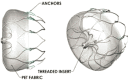
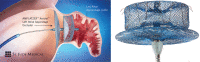
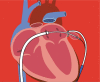
Left atrial appendage occlusion (LAAO) has emerged as an effective site-directed therapy in patients with nonvalvular atrial fibrillation (AF) for stroke prevention, who are ineligible for long-term oral anticoagulation. The objective of this study was to assess the safety, efficacy, and availability of LAAO devices by reviewing the literature and to review the development and effectiveness of LAAO by the transcatheter approach with plugging devices such as WATCHMAN™ (Boston Scientific, Natick, MA, USA); AMPLATZER™ Cardiac Plug and AMPLATZER™ Amulet™ (Abbott Laboratories, Chicago, IL, USA); and the LARIAT® Suture Delivery Device (SentreHEART, Redwood City, CA, USA), which features an entirely unique hybrid (endocardial and epicardial) approach in closing the left atrial appendage (LAA). The conducted literature review ultimately revealed a substantial body of literature supporting the safety and efficacy of various LAAO strategies, including endocardial, epicardial, and hybrid approaches, in AF patients who are not eligible for long-term oral anticoagulant use. Specifically, the most attractive population suitable for LAA closure appears to be patients at high risk for ischemic stroke with a longer life expectancy but a moderate-to-high bleeding risk with long-term oral anticoagulation. The benefit of LAA closure in reducing the incidence of stroke in patients with nonvalvular AF has been evolving gradually, and we are confident that this new field of percutaneous LAA closure will continue to emerge as a game-changer in the treatment of AF.
Keywords: Amulet; WATCHMAN™; anticoagulation; left atrial appendage; thromboembolism.
Copyright: © 2018 Innovations in Cardiac Rhythm Management.
Conflict of interest statement
The authors report no conflicts of interest for the published content. There was no financial involvement of a pharmaceutical or other company in this study.
Figures



References
-
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. [CrossRef] [PubMed] - DOI - PMC - PubMed
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 1991;22(8):983–988. [CrossRef] [PubMed] - DOI - PubMed
-
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. [CrossRef] [PubMed] - DOI - PubMed
-
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000;160(7):967–973. [CrossRef] [PubMed] - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
