Immunogenicity and Reactogenicity of a Reduced Schedule of a 4-component Capsular Group B Meningococcal Vaccine: A Randomized Controlled Trial in Infants
- PMID: 32494580
- PMCID: PMC7252280
- DOI: 10.1093/ofid/ofaa143
Immunogenicity and Reactogenicity of a Reduced Schedule of a 4-component Capsular Group B Meningococcal Vaccine: A Randomized Controlled Trial in Infants
Abstract
Background: The 4-component capsular group B meningococcal vaccine (4CMenB) was licensed as a 4-dose infant schedule but introduced into the United Kingdom as 3 doses at 2, 4, and 12 months of age. We describe the immunogenicity and reactogenicity of the 2 + 1 schedule in infants.
Methods: Infants were randomized to receive 4CMenB with routine immunizations (test group) at 2, 4, and 12 months or 4CMenB alone at 6, 8, and 13 months of age (control group). Serum bactericidal antibody (SBA) assay against a serogroup B meningococcal reference strain (44/76-SL), memory B-cell responses to factor H binding protein, Neisseria adhesion protein A, Neisseria heparin binding antigen, Porin A (PorA), and reactogenicity was measured.
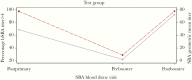
Results: One hundred eighty-seven infants were randomized (test group: 94; control group: 93). In the test group, 4CMenB induced SBA titers above the putative protective threshold (1:4) after primary and booster doses in 97% of participants. Postbooster, the SBA GMT (72.1; 95% confidence interval [CI], 51.7-100.4) was numerically higher than the serum bactericidal antibody geometric mean titre (SBA GMT) determined post-primary vaccination (48.6; 95% CI, 37.2-63.4). After primary immunizations, memory B-cell responses did not change when compared with baseline controls, but frequencies significantly increased after booster. Higher frequency of local and systemic adverse reactions was associated with 4CMenB.
Conclusions: A reduced schedule of 4CMenB was immunogenic and established immunological memory after booster.
Keywords: 4-component capsular group B meningococcal vaccine; 4CMenB; immunogenicity; memory B cells; meningococcal disease; reactogenicity; reduced schedule.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2020.
Figures


References
-
- European Centre for Disease Preventation and Control. Invasive Meningococcal Disease. Annual Epidemiology Report for 2017. Stockholm: ECDC; 2019.
-
- Vesikari T, Esposito S, Prymula R, et al. ; EU Meningococcal B Infant Vaccine Study group Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 2013; 381:825–35. - PubMed
-
- European Medicines Agency. Bexsero: EPAR-product information Available at: http://www.ema.europa.eu2012, accessed in 15/08/2019. Accessed 15 October 2019.
-
- Gossger N, Snape MD, Yu LM, et al. ; European MenB Vaccine Study Group Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 2012; 307:573–82. - PubMed

