A hexavalent Coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates
- PMID: 32494709
- PMCID: PMC7202868
- DOI: 10.1126/sciadv.aaz2433
A hexavalent Coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates
Abstract
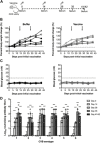
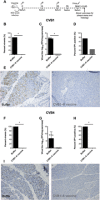
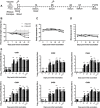
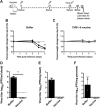
Coxsackievirus B (CVB) enteroviruses are common human pathogens known to cause severe diseases including myocarditis, chronic dilated cardiomyopathy, and aseptic meningitis. CVBs are also hypothesized to be a causal factor in type 1 diabetes. Vaccines against CVBs are not currently available, and here we describe the generation and preclinical testing of a novel hexavalent vaccine targeting the six known CVB serotypes. We show that the vaccine has an excellent safety profile in murine models and nonhuman primates and that it induces strong neutralizing antibody responses to the six serotypes in both species without an adjuvant. We also demonstrate that the vaccine provides immunity against acute CVB infections in mice, including CVB infections known to cause virus-induced myocarditis. In addition, it blocks CVB-induced diabetes in a genetically permissive mouse model. Our preclinical proof-of-concept studies demonstrate the successful generation of a promising hexavalent CVB vaccine with high immunogenicity capable of preventing CVB-induced diseases.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

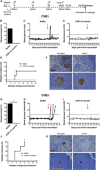
References
-
- Tavakoli N. P., Wang H., Nattanmai S., Dupuis M., Fusco H., Hull R., Detection and typing of enteroviruses from CSF specimens from patients diagnosed with meningitis/encephalitis. J. Clin. Virol. 43, 207–211 (2008). - PubMed
-
- Pollack A., Kontorovich A. R., Fuster V., Dec G. W., Viral myocarditis—Diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 12, 670–680 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

