In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion
- PMID: 32494750
- PMCID: PMC7244313
- DOI: 10.1126/sciadv.aba6357
In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion
Abstract
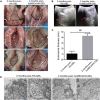
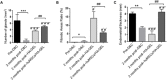
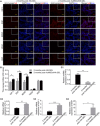
Increasing occurrence of moderate to severe intrauterine adhesion (IUA) is seriously affecting the quality of human life. The aim of the study was to establish IUA models in nonhuman primates and to explore the dual repair effects of human umbilical cord-derived mesenchymal stem cells (huMSCs) loaded on autocrosslinked hyaluronic acid gel (HA-GEL) on endometrial damage and adhesion. Here, we recorded the menstrual cycle data in detail with uterine cavities observed and endometrial tissues detected after intervention, and the thicker endometria, decreased amount of fibrotic formation, increased number of endometrium glands, etc., suggested that both HA-GEL and huMSC/HA-GEL complexes could partially repair IUA caused by mechanical injury, but huMSC/HA-GEL complex transplantation had notable dual repair effects: a reliable antiadhesion property and the promotion of endometrial regeneration.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Evans-Hoeker E. A., Young S. L., Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 32, 392–401 (2014). - PubMed
-
- Hooker A. B., Lemmers M., Thurkow A. L., Heymans M. W., Opmeer B. C., Brölmann H. A. M., Mol B. W., Huirne J. A. F., Systematic review and meta-analysis of intrauterine adhesions after miscarriage: Prevalence, risk factors and long-term reproductive outcome. Hum. Reprod. Update 20, 262–278 (2014). - PubMed
-
- Guo E. J., Chung J. P. W., Poon L. C. Y., Li T. C., Reproductive outcomes after surgical treatment of Asherman syndrome: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 59, 98–114 (2019). - PubMed
-
- Yu D., Wong Y.-M., Cheong Y., Xia E., Li T.-C., Asherman syndrome—One century later. Fertil. Steril. 89, 759–779 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

