Abnormal liver tests in patients hospitalized with Coronavirus disease 2019: Should we worry?
- PMID: 32495496
- PMCID: PMC7300742
- DOI: 10.1111/liv.14557
Abnormal liver tests in patients hospitalized with Coronavirus disease 2019: Should we worry?
Abstract
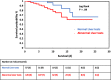
While several studies from China have reported COVID-19-related liver injury, there are currently no data on liver dysfunction in hospitalized COVID-19 patients in Europe. The aim of this study was to describe the prevalence and predictive value of abnormal liver function in patients hospitalized with COVID-19. This was a retrospective cohort study of confirmed COVID-19 patients hospitalized in two referral hospitals in France. Clinical, biological and radiological data were collected and analysed. In all, 234 patients confirmed to have COVID-19 by RT-PCR were included. Liver function was abnormal in 66.6% of patients on admission. In multivariate logistic regression, abnormal liver test on admission were associated with in-hospital aggravation (OR = 4.1, 95% CI 1.5-10.8; P = .004) and mortality (OR 3.3; 95% CI = 1.04-10.5; P = .04). This study of liver tests in a European COVID-19 population confirms a high prevalence of abnormal liver tests on admission that are predictive of severe disease course and higher in-hospital mortality.
Keywords: Coronavirus disease 2019; SARS-CoV-2; abnormal liver tests; in-hospital aggravation; in-hospital mortality; prognosis.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest that pertain to this work.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous