Modifiers of Adeno-Associated Virus-Mediated Gene Expression in Implication for Serotype-Universal Neutralizing Antibody Assay
- PMID: 32495655
- PMCID: PMC7588322
- DOI: 10.1089/hum.2020.074
Modifiers of Adeno-Associated Virus-Mediated Gene Expression in Implication for Serotype-Universal Neutralizing Antibody Assay
Abstract
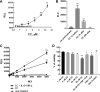
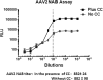
Adeno-associated virus (AAV)-based gene therapy is undergoing major expansion into clinical practice, with two treatments currently being granted Food and Drug Administration (FDA) approval. However, the presence of pre-existing neutralizing antibodies (NAB) is one of the significant hurdles for the clinical application of AAV vectors that significantly limits the patient population, which benefits from the treatment. A reliable diagnostic to evaluate the patient's seropositivity is required to ensure the effectiveness of the AAV-mediated therapeutic. Here, we describe a simple method for the determination of AAV NAB activity based on our finding that Compound C makes HEK293 cell highly permissive for infection by 10 commonly used AAV serotypes.
Keywords: adeno-associated virus; assay development; neutralizing antibodies; selective inhibitor of AMPK.
Conflict of interest statement
K.K. and G.A. hold provisional patents related to the protocol described in the current article. G.A. has several issued patents related to AAV vectors that have been licensed to various gene therapy companies.
Figures
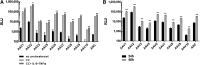

Similar articles
-
Retinal Epithelial Neutralization Assay Optimizes AAV Serotype Selection for Ocular Gene Therapy.Viruses. 2025 Jul 15;17(7):988. doi: 10.3390/v17070988. Viruses. 2025. PMID: 40733604 Free PMC article.
-
Pre-existing antibodies to candidate gene therapy vectors (adeno-associated vector serotypes) in domestic cats.PLoS One. 2019 Mar 21;14(3):e0212811. doi: 10.1371/journal.pone.0212811. eCollection 2019. PLoS One. 2019. PMID: 30897117 Free PMC article.
-
Neutralizing Antibodies Against Adeno-Associated Virus (AAV): Measurement and Influence on Retinal Gene Delivery.Methods Mol Biol. 2018;1715:225-238. doi: 10.1007/978-1-4939-7522-8_16. Methods Mol Biol. 2018. PMID: 29188517
-
Recommendations for the Development of Cell-Based Anti-Viral Vector Neutralizing Antibody Assays.AAPS J. 2020 Jan 6;22(2):24. doi: 10.1208/s12248-019-0403-1. AAPS J. 2020. PMID: 31907680 Review.
-
Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions.Front Immunol. 2021 Mar 17;12:658399. doi: 10.3389/fimmu.2021.658399. eCollection 2021. Front Immunol. 2021. PMID: 33815421 Free PMC article. Review.
Cited by
-
Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy.Mol Ther. 2023 Mar 1;31(3):616-630. doi: 10.1016/j.ymthe.2023.01.010. Epub 2023 Jan 11. Mol Ther. 2023. PMID: 36635967 Free PMC article. Review.
-
Adeno-Associated Viruses (AAV) and Host Immunity - A Race Between the Hare and the Hedgehog.Front Immunol. 2021 Oct 29;12:753467. doi: 10.3389/fimmu.2021.753467. eCollection 2021. Front Immunol. 2021. PMID: 34777364 Free PMC article. Review.
-
Identification of a novel neutralization epitope in rhesus AAVs.Mol Ther Methods Clin Dev. 2024 Oct 4;32(4):101350. doi: 10.1016/j.omtm.2024.101350. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39469420 Free PMC article.
-
Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations.Mol Ther Methods Clin Dev. 2022 Feb 26;25:74-83. doi: 10.1016/j.omtm.2022.02.011. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35356756 Free PMC article. Review.
-
Multiplexing AAV Serotype-Specific Neutralizing Antibodies in Preclinical Animal Models and Humans.Biomedicines. 2023 Feb 11;11(2):523. doi: 10.3390/biomedicines11020523. Biomedicines. 2023. PMID: 36831059 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
