Modifiers of Adeno-Associated Virus-Mediated Gene Expression in Implication for Serotype-Universal Neutralizing Antibody Assay
- PMID: 32495655
- PMCID: PMC7588322
- DOI: 10.1089/hum.2020.074
Modifiers of Adeno-Associated Virus-Mediated Gene Expression in Implication for Serotype-Universal Neutralizing Antibody Assay
Abstract
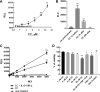
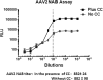
Adeno-associated virus (AAV)-based gene therapy is undergoing major expansion into clinical practice, with two treatments currently being granted Food and Drug Administration (FDA) approval. However, the presence of pre-existing neutralizing antibodies (NAB) is one of the significant hurdles for the clinical application of AAV vectors that significantly limits the patient population, which benefits from the treatment. A reliable diagnostic to evaluate the patient's seropositivity is required to ensure the effectiveness of the AAV-mediated therapeutic. Here, we describe a simple method for the determination of AAV NAB activity based on our finding that Compound C makes HEK293 cell highly permissive for infection by 10 commonly used AAV serotypes.
Keywords: adeno-associated virus; assay development; neutralizing antibodies; selective inhibitor of AMPK.
Conflict of interest statement
K.K. and G.A. hold provisional patents related to the protocol described in the current article. G.A. has several issued patents related to AAV vectors that have been licensed to various gene therapy companies.
Figures
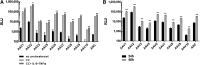

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
