Dynamic topology of double-stranded telomeric DNA studied by single-molecule manipulation in vitro
- PMID: 32496520
- PMCID: PMC7337930
- DOI: 10.1093/nar/gkaa479
Dynamic topology of double-stranded telomeric DNA studied by single-molecule manipulation in vitro
Abstract
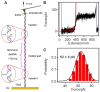
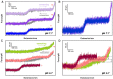
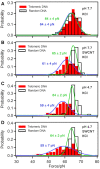
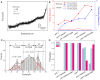
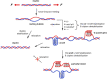
The dynamic topological structure of telomeric DNA is closely related to its biological function; however, no such structural information on full-length telomeric DNA has been reported due to difficulties synthesizing long double-stranded telomeric DNA. Herein, we developed an EM-PCR and TA cloning-based approach to synthesize long-chain double-stranded tandem repeats of telomeric DNA. Using mechanical manipulation assays based on single-molecule atomic force microscopy, we found that mechanical force can trigger the melting of double-stranded telomeric DNA and the formation of higher-order structures (G-quadruplexes or i-motifs). Our results show that only when both the G-strand and C-strand of double-stranded telomeric DNA form higher-order structures (G-quadruplexes or i-motifs) at the same time (e.g. in the presence of 100 mM KCl under pH 4.7), that the higher-order structure(s) can remain after the external force is removed. The presence of monovalent K+, single-wall carbon nanotubes (SWCNTs), acidic conditions, or short G-rich fragments (∼30 nt) can shift the transition from dsDNA to higher-order structures. Our results provide a new way to regulate the topology of telomeric DNA.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

