Differential Antibody Recognition of H3N2 Vaccine and Seasonal Influenza Virus Strains Based on Age, Vaccine Status, and Sex in the 2017-2018 Season
- PMID: 32496543
- PMCID: PMC7488199
- DOI: 10.1093/infdis/jiaa289
Differential Antibody Recognition of H3N2 Vaccine and Seasonal Influenza Virus Strains Based on Age, Vaccine Status, and Sex in the 2017-2018 Season
Abstract
Background: An antigenic mismatch between the vaccine and circulating H3N2 strains was hypothesized to contribute to the severity of the 2017-2018 season in North America.
Methods: Serum and nasal washes were collected from influenza positive and negative patients during the 2017-2018 season to determine neutralizing antibody (nAb) titers and for influenza virus sequencing, respectively.
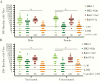
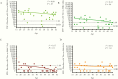
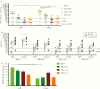
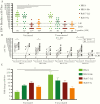
Results: The circulating and vaccine H3N2 virus strains were different clades, with the vaccine strain being clade 3C.2a and the circulating viruses being 3C.2a2 or 3C.3a. At enrollment, both the H3N2 negative and positive patients had greater nAb titers to the egg-adapted vaccine virus compared to the cell-grown vaccine but the H3N2-negative population had significantly greater titers to the circulating 3C.2a2. Among H3N2-positive patients, vaccination, younger age, and female sex were associated with greater nAb responses to the egg-adapted vaccine H3N2 virus but not to the cell-grown vaccine or circulating viruses.
Conclusions: For the 2017-2018 circulating viruses, mutations introduced by egg adaptation decreased vaccine efficacy. No increased protection was afforded by vaccination, younger age, or female sex against 2017-2018 circulating H3N2 viruses.
Keywords: circulating influenza strain; egg adaptation; human surveillance; neutralizing antibody; seasonal influenza vaccine; sex differences.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses.PLoS One. 2014 Mar 25;9(3):e92153. doi: 10.1371/journal.pone.0092153. eCollection 2014. PLoS One. 2014. PMID: 24667168 Free PMC article.
-
Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):12578-12583. doi: 10.1073/pnas.1712377114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109276 Free PMC article.
-
In-depth phylogenetic analysis of the hemagglutinin gene of influenza A(H3N2) viruses circulating during the 2016-2017 season revealed egg-adaptive mutations of vaccine strains.Expert Rev Vaccines. 2020 Jan;19(1):115-122. doi: 10.1080/14760584.2020.1709827. Epub 2020 Jan 19. Expert Rev Vaccines. 2020. PMID: 31875483
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
-
Improving the selection and development of influenza vaccine viruses - Report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18-20 November 2015.Vaccine. 2017 Feb 22;35(8):1104-1109. doi: 10.1016/j.vaccine.2017.01.018. Epub 2017 Jan 25. Vaccine. 2017. PMID: 28131392 Free PMC article. Review.
Cited by
-
Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination.J Clin Invest. 2021 Apr 15;131(8):e146138. doi: 10.1172/JCI146138. J Clin Invest. 2021. PMID: 33690218 Free PMC article.
-
Early transcriptional responses of human nasal epithelial cells to infection with Influenza A and SARS-CoV-2 virus differ and are influenced by physiological temperature.bioRxiv [Preprint]. 2023 Mar 9:2023.03.07.531609. doi: 10.1101/2023.03.07.531609. bioRxiv. 2023. Update in: Pathogens. 2023 Mar 18;12(3):480. doi: 10.3390/pathogens12030480. PMID: 36945583 Free PMC article. Updated. Preprint.
-
Biological Sex and Pregnancy Affect Influenza Pathogenesis and Vaccination.Curr Top Microbiol Immunol. 2023;441:111-137. doi: 10.1007/978-3-031-35139-6_5. Curr Top Microbiol Immunol. 2023. PMID: 37695427
-
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial.JCI Insight. 2024 Mar 14;9(8):e178460. doi: 10.1172/jci.insight.178460. JCI Insight. 2024. PMID: 38483534 Free PMC article. Clinical Trial.
-
Early Transcriptional Responses of Human Nasal Epithelial Cells to Infection with Influenza A and SARS-CoV-2 Virus Differ and Are Influenced by Physiological Temperature.Pathogens. 2023 Mar 18;12(3):480. doi: 10.3390/pathogens12030480. Pathogens. 2023. PMID: 36986402 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Influenza (flu). Vaccine supply for 2019–2020 season https://www.cdc.gov/flu/prevent/vaxsupply.htm?CDC_AA_refVal=https%3A%2F%.... Accessed 15 October 15 2019.
-
- Shrestha SS, Swerdlow DL, Borse RH, et al. . Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009-April 2010). Clin Infect Dis 2011; 52 (suppl 1:S75–82. - PubMed
-
- Centers for Disease Control and Prevention. Influenza (flu). Estimated influenza illness, medical visits, hospitalizationsm and deaths in the United States—2017–2018 influenza season.https://www.cdc.gov/flu/about/burden/2017-2018.htm. Accessed 15 October 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

