Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study
- PMID: 32496550
- PMCID: PMC7273318
- DOI: 10.1001/jamaoncol.2020.1271
Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study
Erratum in
-
Middle Initial Omitted From Author Name.JAMA Oncol. 2020 Sep 1;6(9):1473. doi: 10.1001/jamaoncol.2020.3404. JAMA Oncol. 2020. PMID: 32701125 Free PMC article. No abstract available.
Abstract
Importance: Although stereotactic radiosurgery (SRS) is preferred for limited brain metastases from most histologies, whole-brain radiotherapy (WBRT) has remained the standard of care for patients with small cell lung cancer. Data on SRS are limited.
Objective: To characterize and compare first-line SRS outcomes (without prior WBRT or prophylactic cranial irradiation) with those of first-line WBRT.
Design, setting, and participants: FIRE-SCLC (First-line Radiosurgery for Small-Cell Lung Cancer) was a multicenter cohort study that analyzed SRS outcomes from 28 centers and a single-arm trial and compared these data with outcomes from a first-line WBRT cohort. Data were collected from October 26, 2017, to August 15, 2019, and analyzed from August 16, 2019, to November 6, 2019.
Interventions: SRS and WBRT for small cell lung cancer brain metastases.
Main outcomes and measures: Overall survival, time to central nervous system progression (TTCP), and central nervous system (CNS) progression-free survival (PFS) after SRS were evaluated and compared with WBRT outcomes, with adjustment for performance status, number of brain metastases, synchronicity, age, sex, and treatment year in multivariable and propensity score-matched analyses.
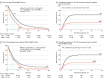
Results: In total, 710 patients (median [interquartile range] age, 68.5 [62-74] years; 531 men [74.8%]) who received SRS between 1994 and 2018 were analyzed. The median overall survival was 8.5 months, the median TTCP was 8.1 months, and the median CNS PFS was 5.0 months. When stratified by the number of brain metastases treated, the median overall survival was 11.0 months (95% CI, 8.9-13.4) for 1 lesion, 8.7 months (95% CI, 7.7-10.4) for 2 to 4 lesions, 8.0 months (95% CI, 6.4-9.6) for 5 to 10 lesions, and 5.5 months (95% CI, 4.3-7.6) for 11 or more lesions. Competing risk estimates were 7.0% (95% CI, 4.9%-9.2%) for local failures at 12 months and 41.6% (95% CI, 37.6%-45.7%) for distant CNS failures at 12 months. Leptomeningeal progression (46 of 425 patients [10.8%] with available data) and neurological mortality (80 of 647 patients [12.4%] with available data) were uncommon. On propensity score-matched analyses comparing SRS with WBRT, WBRT was associated with improved TTCP (hazard ratio, 0.38; 95% CI, 0.26-0.55; P < .001), without an improvement in overall survival (median, 6.5 months [95% CI, 5.5-8.0] for SRS vs 5.2 months [95% CI, 4.4-6.7] for WBRT; P = .003) or CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79). Multivariable analyses comparing SRS and WBRT, including subset analyses controlling for extracranial metastases and extracranial disease control status, demonstrated similar results.
Conclusions and relevance: Results of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP without a decrease in overall survival, are similar to those observed in settings in which SRS is already established.
Conflict of interest statement
Figures

Comment in
-
Radiosurgery in Patients With Small Cell Lung Cancer With Brain Metastases: A Call for Prospective Evidence.JAMA Oncol. 2020 Jul 1;6(7):1037-1038. doi: 10.1001/jamaoncol.2020.1245. JAMA Oncol. 2020. PMID: 32496551 No abstract available.
-
Deferring a Change in the Standard of Care for Small Cell Lung Cancer Brain Metastases-Reply.JAMA Oncol. 2021 Jan 1;7(1):135-136. doi: 10.1001/jamaoncol.2020.5473. JAMA Oncol. 2021. PMID: 33180104 No abstract available.
-
Deferring a Change in the Standard of Care for Small Cell Lung Cancer Brain Metastases.JAMA Oncol. 2021 Jan 1;7(1):134-135. doi: 10.1001/jamaoncol.2020.5466. JAMA Oncol. 2021. PMID: 33180110 No abstract available.

