Subtherapeutic Acetazolamide Doses as a Noninvasive Method for Assessing Medication Adherence
- PMID: 32496573
- PMCID: PMC7669583
- DOI: 10.1002/cpt.1929
Subtherapeutic Acetazolamide Doses as a Noninvasive Method for Assessing Medication Adherence
Abstract
Adherence monitoring is a vital component of clinical efficacy trials, as the regularity of medication consumption affects both efficacy and adverse effect profiles. Pill-counts do not confirm consumption, and invasive plasma assessments can only assist post hoc assessments. We previously reported on the pharmacokinetics of a potential adherence marker to noninvasively monitor dosage consumption during a trial without breaking a blind. We reported that consumption cessation of subtherapeutic 15 mg acetazolamide (ACZ) doses showed a predictable urinary excretion decay that was quantifiable for an extended period. The current study describes the clinical implementation of 15 mg ACZ doses as an adherence marker excipient in distinct cohorts taking ACZ for different "adherence" durations. We confirm that ACZ output did not change (accumulate) during 18-20 days of adherence, and developed and assessed urinary cutoffs as nonadherence indicators. We demonstrate that whereas an absolute concentration cutoff (989 ng/mL) lacked sensitivity, a creatinine normalized equivalent (1,376 ng/mg ACZ) was highly accurate at detecting nonadherence. We also demonstrate that during nonadherent phases of three trials, creatinine-normalized urinary ACZ elimination was reproducible within and across trials with low variability. Excretion was first order, with a decay half-life averaging ~ 2.0 days. Further, excretion remained quantifiable for 14 days, providing a long period during which the date of last consumption might be determined. We conclude that inclusion of 15 mg ACZ as a dosage form adherence marker excipient, provides a reliable and sensitive mechanism to confirm medication consumption and detect nonadherence during clinical efficacy trials.
Published 2020. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest
The authors declared no competing interests for this work.
Figures
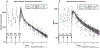

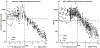

References
-
- Zempleni J, Galloway JR & McCormick DB Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. The American journal of clinical nutrition 63, 54–66 (1996). - PubMed
-
- Granero GE et al. Biowaiver monographs for immediate release solid oral dosage forms: acetazolamide. Journal of pharmaceutical sciences 97, 3691–9 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

