Assessment of vestibulocortical interactions during standing in healthy subjects
- PMID: 32497147
- PMCID: PMC7272097
- DOI: 10.1371/journal.pone.0233843
Assessment of vestibulocortical interactions during standing in healthy subjects
Abstract
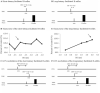
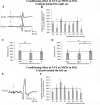
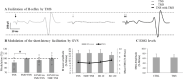
The vestibular system is essential to produce adequate postural responses enabling voluntary movement. However, how the vestibular system influences corticospinal output during postural tasks is still unknown. Here, we examined the modulation exerted by the vestibular system on corticospinal output during standing. Healthy subjects (n = 25) maintained quiet standing, head facing forward with eyes closed. Galvanic vestibular stimulation (GVS) was applied bipolarly and binaurally at different delays prior to transcranial magnetic stimulation (TMS) which triggered motor evoked potentials (MEPs). With the cathode right/anode left configuration, MEPs in right Soleus (SOL) muscle were significantly suppressed when GVS was applied at ISI = 40 and 130ms before TMS. With the anode right/cathode left configuration, no significant changes were observed. Changes in the MEP amplitude were then compared to changes in the ongoing EMG when GVS was applied alone. Only the decrease in MEP amplitude at ISI = 40ms occurred without change in the ongoing EMG, suggesting that modulation occurred at a premotoneuronal level. We further investigated whether vestibular modulation could occur at the motor cortex level by assessing changes in the direct corticospinal pathways using the short-latency facilitation of the SOL Hoffmann reflex (H-reflex) by TMS. None of the observed modulation occurred at the level of motor cortex. Finally, using the long-latency facilitation of the SOL H-reflex, we were able to confirm that the suppression of MEP at ISI = 40ms occurred at a premotoneuronal level. The data indicate that vestibular signals modulate corticospinal output to SOL at both premotoneuronal and motoneuronal levels during standing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

