Intratumoral CD4+ T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer
- PMID: 32497499
- PMCID: PMC7321885
- DOI: 10.1016/j.cell.2020.05.017
Intratumoral CD4+ T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer
Abstract
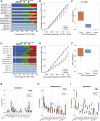
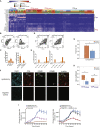
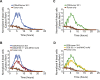
Responses to anti-PD-1 immunotherapy occur but are infrequent in bladder cancer. The specific T cells that mediate tumor rejection are unknown. T cells from human bladder tumors and non-malignant tissue were assessed with single-cell RNA and paired T cell receptor (TCR) sequencing of 30,604 T cells from 7 patients. We find that the states and repertoires of CD8+ T cells are not distinct in tumors compared with non-malignant tissues. In contrast, single-cell analysis of CD4+ T cells demonstrates several tumor-specific states, including multiple distinct states of regulatory T cells. Surprisingly, we also find multiple cytotoxic CD4+ T cell states that are clonally expanded. These CD4+ T cells can kill autologous tumors in an MHC class II-dependent fashion and are suppressed by regulatory T cells. Further, a gene signature of cytotoxic CD4+ T cells in tumors predicts a clinical response in 244 metastatic bladder cancer patients treated with anti-PD-L1.
Trial registration: ClinicalTrials.gov NCT02451423.
Keywords: Bladder cancer; PD-1 blockade; anti-PD-L1; checkpoint inhibition; cytotoxic CD4(+) T cells; predictive gene signature; single-cell sequencing.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests D.Y.O. has received research support from Roche/Genentech and Merck and has served as a paid consultant for Maze Therapeutics. L.F. has received research support from Roche/Genentech, Abbvie, Bavarian Nordic, Bristol Myers Squibb, Janssen, and Merck. C.J.Y. is a co-founder of Dropprint Genomics.
Figures







Comment in
-
Cytotoxic CD4+ T Cells in Bladder Cancer-A New License to Kill.Cancer Cell. 2020 Jul 13;38(1):28-30. doi: 10.1016/j.ccell.2020.06.013. Cancer Cell. 2020. PMID: 32663467
-
Uro-Science.J Urol. 2021 Apr;205(4):1228-1229. doi: 10.1097/JU.0000000000001605. Epub 2021 Jan 21. J Urol. 2021. PMID: 33472375 No abstract available.
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles Of Oncology.J Urol. 2021 Dec;206(6):1517-1519. doi: 10.1097/JU.0000000000002213. Epub 2021 Sep 8. J Urol. 2021. PMID: 34494452 No abstract available.
References
-
- Baras A.S., Drake C., Liu J.J., Gandhi N., Kates M., Hoque M.O., Meeker A., Hahn N., Taube J.M., Schoenberg M.P. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. OncoImmunology. 2016;5:e1134412. - PMC - PubMed
-
- Bolotin D.A., Poslavsky S., Mitrophanov I., Shugay M., Mamedov I.Z., Putintseva E.V., Chudakov D.M. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods. 2015;12:380–381. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

