Review of methods for detecting glycemic disorders
- PMID: 32497744
- PMCID: PMC7977482
- DOI: 10.1016/j.diabres.2020.108233
Review of methods for detecting glycemic disorders
Erratum in
-
Correction to the Bergman review.Diabetes Res Clin Pract. 2021 Oct;180:108632. doi: 10.1016/j.diabres.2020.108632. Epub 2020 Dec 17. Diabetes Res Clin Pract. 2021. PMID: 33346071 No abstract available.
Abstract
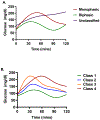
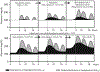
Prediabetes (intermediate hyperglycemia) consists of two abnormalities, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) detected by a standardized 75-gram oral glucose tolerance test (OGTT). Individuals with isolated IGT or combined IFG and IGT have increased risk for developing type 2 diabetes (T2D) and cardiovascular disease (CVD). Diagnosing prediabetes early and accurately is critical in order to refer high-risk individuals for intensive lifestyle modification. However, there is currently no international consensus for diagnosing prediabetes with HbA1c or glucose measurements based upon American Diabetes Association (ADA) and the World Health Organization (WHO) criteria that identify different populations at risk for progressing to diabetes. Various caveats affecting the accuracy of interpreting the HbA1c including genetics complicate this further. This review describes established methods for detecting glucose disorders based upon glucose and HbA1c parameters as well as novel approaches including the 1-hour plasma glucose (1-h PG), glucose challenge test (GCT), shape of the glucose curve, genetics, continuous glucose monitoring (CGM), measures of insulin secretion and sensitivity, metabolomics, and ancillary tools such as fructosamine, glycated albumin (GA), 1,5- anhydroglucitol (1,5-AG). Of the approaches considered, the 1-h PG has considerable potential as a biomarker for detecting glucose disorders if confirmed by additional data including health economic analysis. Whether the 1-h OGTT is superior to genetics and omics in providing greater precision for individualized treatment requires further investigation. These methods will need to demonstrate substantially superiority to simpler tools for detecting glucose disorders to justify their cost and complexity.
Keywords: Biomarkers; Cardiovascular disease; Continuous glucose monitoring; Glycemic variability; HbA1c; Metabolomics; Oral glucose tolerance test; Prediabetes; Type 2 diabetes.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest We declare no competing interests.
Figures

References
-
- Federation ID. IDF Diabetes Atlas. 8 ed. Brussels, Belgium: International Diabetes Federation; 2017.
-
- Makaroff LE. The need for international consensus on prediabetes. Lancet Diabetes Endocrinol 2017;5:5–7. - PubMed
-
- Cohen RM, Franco RS, Smith EP, Higgins JM. When HbA1c and Blood Glucose Do Not Match: How Much Is Determined by Race, by Genetics, by Differences in Mean Red Blood Cell Age?. J Clin Endocrinol Metabol 2019;104:707–10. - PubMed
-
- Nayak AU, Singh BM, Dunmore SJ. Potential Clinical Error Arising From Use of HbA1c in Diabetes: Effects of the Glycation Gap. Endocr Rev 2019;40:988–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 CX001025/CX/CSRD VA/United States
- R03 AI133172/AI/NIAID NIH HHS/United States
- U01 DK098246/DK/NIDDK NIH HHS/United States
- R01 DK107680/DK/NIDDK NIH HHS/United States
- I01 CX001737/CX/CSRD VA/United States
- R21 DK099716/DK/NIDDK NIH HHS/United States
- U34 DK091958/DK/NIDDK NIH HHS/United States
- P30 DK111024/DK/NIDDK NIH HHS/United States
- R01 DK024092/DK/NIDDK NIH HHS/United States
- IK2 RX002928/RX/RRD VA/United States
- R01 DK097554/DK/NIDDK NIH HHS/United States
- T32 HL098129/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

