Combination of thrombolytic and immunosuppressive therapy for coronavirus disease 2019: A case report
- PMID: 32497796
- PMCID: PMC7263262
- DOI: 10.1016/j.ijid.2020.05.118
Combination of thrombolytic and immunosuppressive therapy for coronavirus disease 2019: A case report
Abstract
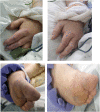
In a proportion of patients, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a multisystem syndrome characterized by hyperinflammation, acute respiratory distress syndrome (ARDS), and hypercoagulability. A 68-year-old man with coronavirus disease 2019 (COVID-19) was admitted to the intensive care unit with respiratory failure, cytokine release syndrome (CRS), and skin ischemia - microthrombosis. Specific coagulation and inflammatory markers (D-dimer, ferritin, and C-reactive protein), along with the clinical picture, triggered the trial of recombinant tissue plasminogen activator (rt-PA) and tocilizumab. This was followed by resolution of the skin ischemia and CRS, while respiratory parameters improved. No major complications associated with rt-PA or tocilizumab occurred. The combination of rt-PA with targeted anti-inflammatory treatment could be a new therapeutic option for patients with COVID-19, ARDS, hyperinflammation, and increased blood viscosity.
Keywords: Acute respiratory distress syndrome; Coronavirus disease 2019; D-dimer; Recombinant tissue plasminogen activator; Tocilizumab.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Available at: https://www.who.int/ publications-detail /clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed April 10.
-
- Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526–2533. - PubMed
-
- Fardet L., Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. - PubMed
-
- Moore B.H., Barrett D.C., Moore E.E., McIntyre C.R., Moore K.P., Talmor S.D. Is There a Role for Tissue Plasminogen Activator (tPA) as a Novel Treatment for Refractory COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS)? Journal of Trauma and Acute Care Surgery. 2020 Publish Ahead of Print. - PMC - PubMed
-
- Xiaoling X., Tiantian L., Wei S., Wei S., Dongsheng W., Binqing F. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv [Internet]. 2020 Available at: https://www. ser.es/wp-content/uploads/2020 /03/TCZ-and-COVID-19.pdf. Accessed April 11, 2020. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

