Imbalance in T follicular helper cells producing IL-17 promotes pro-inflammatory responses in MuSK antibody positive myasthenia gravis
- PMID: 32497931
- PMCID: PMC7397478
- DOI: 10.1016/j.jneuroim.2020.577279
Imbalance in T follicular helper cells producing IL-17 promotes pro-inflammatory responses in MuSK antibody positive myasthenia gravis
Abstract
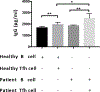
A detailed understanding of the role of Tfh cells in MuSK-antibody positive myasthenia gravis (MuSK-MG) is lacking. We characterized phenotype and function of Tfh cells in MuSK-MG patients and controls. We found similar overall Tfh and follicular regulatory (Tfr) T cell frequencies in MuSK-MG and healthy controls, but MuSK-MG patients exhibited higher frequencies of Tfh17 cells and a higher ratio of Tfh:Tfr cells. These results suggest imbalanced Tfh cell regulation, further supported by increased frequencies of CD4 T cells co-producing IL-21/IL-17 and IL-17/IFN-γ, and increased Tfh-supported IgG production. These results support a role for Tfh cell dysregulation in MuSK-MG immunopathology.
Keywords: Autoimmunity; MuSK antibody; Myasthenia gravis; T follicular helper cells; Tfh17 cells.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures




References
-
- Bentebibel S-E, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H, 2013. Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination. Sci. Transl. Med 5, 176ra132–176ra132. 10.1126/scitranslmed.3005191. - DOI - PMC - PubMed
-
- Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, Wloka K, Wheatley A, Narpala S, McDermott A, Roederer M, Haubrich R, Connors M, Ake J, Douek DC, Kim J, Petrovas C, Koup RA, 2014. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 10, e1003853 10.1371/journal.ppat.1003853. - DOI - PMC - PubMed
-
- Brenna E, Davydov AN, Ladell K, McLaren JE, Bonaiuti P, Metsger M, Ramsden JD, Gilbert SC, Lambe T, Price DA, Campion SL, Chudakov DM, Borrow P, McMichael AJ, 2020. CD4(+) T Follicular Helper Cells in Human Tonsils and Blood Are Clonally Convergent but Divergent from Non-Tfh CD4(+) Cells. Cell reports. 30, 137–152.e135. 10.1016/j.celrep.2019.12.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

