Unleashing the Diagnostic, Prognostic and Therapeutic Potential of the Neuronostatin/GPR107 System in Prostate Cancer
- PMID: 32498336
- PMCID: PMC7355908
- DOI: 10.3390/jcm9061703
Unleashing the Diagnostic, Prognostic and Therapeutic Potential of the Neuronostatin/GPR107 System in Prostate Cancer
Abstract
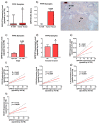
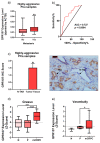
Certain components of the somatostatin-system play relevant roles in Prostate Cancer (PCa), whose most aggressive phenotype (Castration-Resistant-PCa (CRPC)) remains lethal nowadays. However, neuronostatin and the G protein-coupled receptor 107 (GPR107), two novel members of the somatostatin-system, have not been explored yet in PCa. Consequently, we investigated the pathophysiological role of NST/GPR107-system in PCa. GPR107 expression was analyzed in well-characterized PCa patient's cohorts, and functional/mechanistic assays were performed in response to GPR107-silencing and NST-treatment in PCa cells (androgen-dependent (AD: LNCaP) and androgen-independent (AI: 22Rv1/PC-3), which are cell models of hormone-sensitive and CRPC, respectively), and normal prostate cells (RWPE-1 cell-line). GPR107 was overexpressed in PCa and associated with key clinical parameters (e.g., advance stage of PCa, presence of vascular invasion and metastasis). Furthermore, GPR107-silencing inhibited proliferation/migration rates in AI-PCa-cells and altered key genes and oncogenic signaling-pathways involved in PCa aggressiveness (i.e., KI67/CDKN2D/MMP9/PRPF40A, SST5TMD4/AR-v7/In1-ghrelin/EZH2 splicing-variants and AKT-signaling). Interestingly, NST treatment inhibited proliferation/migration only in AI-PCa cells and evoked an identical molecular response than GPR107-silencing. Finally, NST decreased GPR107 expression exclusively in AI-PCa-cells, suggesting that part of the specific antitumor effects of NST could be mediated through a GPR107-downregulation. Altogether, NST/GPR107-system could represent a valuable diagnostic and prognostic tool and a promising novel therapeutic target for PCa and CRPC.
Keywords: G protein-coupled receptor GPR107; castration resistant prostate cancer; diagnostic/prognostic biomarker; neuronostatin; prostate cancer; somatostatin-system; splicing; therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Somatostatin, Cortistatin and Their Receptors Exert Antitumor Actions in Androgen-Independent Prostate Cancer Cells: Critical Role of Endogenous Cortistatin.Int J Mol Sci. 2022 Oct 27;23(21):13003. doi: 10.3390/ijms232113003. Int J Mol Sci. 2022. PMID: 36361790 Free PMC article.
-
Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer.EBioMedicine. 2020 Jan;51:102547. doi: 10.1016/j.ebiom.2019.11.008. Epub 2020 Jan 3. EBioMedicine. 2020. PMID: 31902674 Free PMC article.
-
The oncogenic role of the In1-ghrelin splicing variant in prostate cancer aggressiveness.Mol Cancer. 2017 Aug 29;16(1):146. doi: 10.1186/s12943-017-0713-9. Mol Cancer. 2017. PMID: 28851363 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Targeting Inflammatory Signaling in Prostate Cancer Castration Resistance.J Clin Med. 2021 Oct 27;10(21):5000. doi: 10.3390/jcm10215000. J Clin Med. 2021. PMID: 34768524 Free PMC article. Review.
Cited by
-
Somatostatin, Cortistatin and Their Receptors Exert Antitumor Actions in Androgen-Independent Prostate Cancer Cells: Critical Role of Endogenous Cortistatin.Int J Mol Sci. 2022 Oct 27;23(21):13003. doi: 10.3390/ijms232113003. Int J Mol Sci. 2022. PMID: 36361790 Free PMC article.
-
Dysregulation of the miRNome unveils a crosstalk between obesity and prostate cancer: miR-107 asa personalized diagnostic and therapeutic tool.Mol Ther Nucleic Acids. 2022 Feb 12;27:1164-1178. doi: 10.1016/j.omtn.2022.02.010. eCollection 2022 Mar 8. Mol Ther Nucleic Acids. 2022. PMID: 35282415 Free PMC article.
-
The Use of Biologics for Targeting GPCRs in Metastatic Cancers.BioTech (Basel). 2025 Jan 30;14(1):7. doi: 10.3390/biotech14010007. BioTech (Basel). 2025. PMID: 39982274 Free PMC article. Review.
References
-
- Quagliariello V., Rossetti S., Cavaliere C., Di Palo R., Lamantia E., Castaldo L., Nocerino F., Ametrano G., Cappuccio F., Malzone G., et al. Metabolic syndrome, endocrine disruptors and prostate cancer associations: Biochemical and pathophysiological evidences. Oncotarget. 2017;8:30606–30616. doi: 10.18632/oncotarget.16725. - DOI - PMC - PubMed
-
- Fizazi K., Scher H.I., Molina A., Logothetis C.J., Chi K.N., Jones R.J., Staffurth J.N., North S., Vogelzang N.J., Saad F., et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

