Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study
- PMID: 32498337
- PMCID: PMC7352859
- DOI: 10.3390/nu12061650
Heightened Plasma Levels of Transforming Growth Factor Beta (TGF-β) and Increased Degree of Systemic Biochemical Perturbation Characterizes Hepatic Steatosis in Overweight Pediatric Patients: A Cross-Sectional Study
Abstract
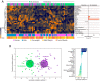
Nonalcoholic Fatty Liver Disease (NAFLD) is a common cause of chronic liver disease in childhood and strongly associated with obesity. Routine biochemical non-invasive tests remain with low accuracy for diagnosis of NAFLD. We performed a cross-sectional study to examine potential associations between anthropometric and biochemical parameters, specially TGF-β, a prognosis marker for hepatic steatosis (HS). Between May and October 2019, seventy-two overweight adolescents were enrolled, of which 36 had hepatic steatosis. Hepatic, lipidic and glycemic profiles, and levels of vitamin D, ferritin and TGF-β were analyzed. Hierarchical cluster and a discriminant model using canonical correlations were employed to depict the overall expression profile of biochemical markers and the biochemical degree of perturbation. Median values of alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), and TGF-β were higher in the adolescents with HS. Values of body mass index (BMI)/age and ALT, but not of TGF-β, were gradually increased proportionally to augmentation of steatosis severity. In a multivariate analysis, TGF-β plasma concentrations were associated with occurrence of hepatic steatosis independent of other covariates. Discriminant analysis confirmed that TGF-β concentrations can identify HS cases. Our data reveal that HS patients exhibit a distinct biosignature of biochemical parameters and imply TGF-β as an important biomarker to evaluate risk of steatosis development.
Keywords: cross-sectional studies; non-alcoholic fatty liver disease; pediatric obesity; systemic biochemical perturbation; transaminases; transforming growth factor beta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Vos M.B., Abrams S.H., Barlow S.E., Caprio S., Daniels S.R., Kohli R., Mouzaki M., Sathya P., Schwimmer J.B., Sundaram S.S., et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J. Pediatr. Gastroenterol. Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

