Comparative Molecular Transporter Properties of Cyclic Peptides Containing Tryptophan and Arginine Residues Formed through Disulfide Cyclization
- PMID: 32498339
- PMCID: PMC7321319
- DOI: 10.3390/molecules25112581
Comparative Molecular Transporter Properties of Cyclic Peptides Containing Tryptophan and Arginine Residues Formed through Disulfide Cyclization
Abstract
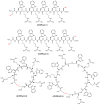
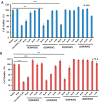
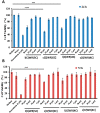
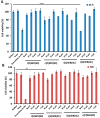
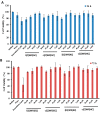
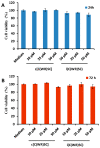
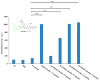
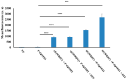
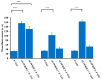
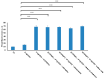
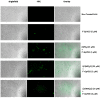
We have previously reported cyclic cell-penetrating peptides [WR]5 and [WR]4 as molecular transporters. To optimize further the utility of our developed peptides for targeted therapy in cancer cells using the redox condition, we designed a new generation of peptides and evaluated their cytotoxicity as well as uptake behavior against different cancer cell lines. Thus, cyclic [C(WR)xC] and linear counterparts (C(WR)xC), where x = 4-5, were synthesized using Fmoc/tBu solid-phase peptide synthesis, purified, and characterized. The compounds did not show any significant cytotoxicity (at 25 µM) against ovarian (SK-OV-3), leukemia (CCRF-CEM), gastric adenocarcinoma (CRL-1739), breast carcinoma (MDA-MB-231), and normal kidney (LLCPK) cells after 24 and 72 h incubation. Both cyclic [C(WR)5C] and linear (C(WR)5C) demonstrated comparable molecular transporter properties versus [WR]5 in the delivery of a phosphopeptide (F'-GpYEEI) in CCRF-CEM cells. The uptake of F'-GpYEEI in the presence of 1,4-dithiothreitol (DTT) as the reducing agent was significantly improved in case of l(C(WR)5C), while it was not changed by [C(WR)5C]. Fluorescence microscopy also demonstrated a significant uptake of F'-GpYEEI in the presence of l(C(WR)5C). Cyclic [C(WR)5C] improved the uptake of the fluorescent-labeled anti-HIV drugs F'-d4T, F'-3TC, and F'-FTC by 3.0-4.9-fold. These data indicate that both [C(WR)5C] and linear (C(WR)5C) peptides can act as molecular transporters.
Keywords: cancer; cell-penetrating peptide; cellular uptake; cytotoxicity; disulfide bridge; drug delivery; phosphopeptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hybrid Cyclic-Linear Cell-Penetrating Peptides Containing Alternative Positively Charged and Hydrophobic Residues as Molecular Transporters.Mol Pharm. 2021 Oct 4;18(10):3909-3919. doi: 10.1021/acs.molpharmaceut.1c00594. Epub 2021 Sep 7. Mol Pharm. 2021. PMID: 34491768
-
Amphiphilic Cell-Penetrating Peptides Containing Natural and Unnatural Amino Acids as Drug Delivery Agents.Cells. 2022 Mar 29;11(7):1156. doi: 10.3390/cells11071156. Cells. 2022. PMID: 35406720 Free PMC article.
-
Comparative Molecular Transporter Efficiency of Cyclic Peptides Containing Tryptophan and Arginine Residues.ACS Omega. 2018 Nov 29;3(11):16281-16291. doi: 10.1021/acsomega.8b02589. eCollection 2018 Nov 30. ACS Omega. 2018. PMID: 31458264 Free PMC article.
-
The rational design of cell-penetrating peptides for application in delivery systems.Peptides. 2019 Nov;121:170149. doi: 10.1016/j.peptides.2019.170149. Epub 2019 Sep 3. Peptides. 2019. PMID: 31491454 Review.
-
Flexible or fixed: a comparative review of linear and cyclic cancer-targeting peptides.Future Med Chem. 2012 Aug;4(12):1601-18. doi: 10.4155/fmc.12.75. Future Med Chem. 2012. PMID: 22917248 Review.
Cited by
-
Exploring the Chemical Features and Biomedical Relevance of Cell-Penetrating Peptides.Int J Mol Sci. 2024 Dec 25;26(1):59. doi: 10.3390/ijms26010059. Int J Mol Sci. 2024. PMID: 39795918 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

