Cancer Biology and Prevention in Diabetes
- PMID: 32498358
- PMCID: PMC7349292
- DOI: 10.3390/cells9061380
Cancer Biology and Prevention in Diabetes
Abstract
The available evidence suggests a complex relationship between diabetes and cancer. Epidemiological data suggest a positive correlation, however, in certain types of cancer, a more complex picture emerges, such as in some site-specific cancers being specific to type I diabetes but not to type II diabetes. Reports share common and differential mechanisms which affect the relationship between diabetes and cancer. We discuss the use of antidiabetic drugs in a wide range of cancer therapy and cancer therapeutics in the development of hyperglycemia, especially antineoplastic drugs which often induce hyperglycemia by targeting insulin/IGF-1 signaling. Similarly, dipeptidyl peptidase 4 (DPP-4), a well-known target in type II diabetes mellitus, has differential effects on cancer types. Past studies suggest a protective role of DPP-4 inhibitors, but recent studies show that DPP-4 inhibition induces cancer metastasis. Moreover, molecular pathological mechanisms of cancer in diabetes are currently largely unclear. The cancer-causing mechanisms in diabetes have been shown to be complex, including excessive ROS-formation, destruction of essential biomolecules, chronic inflammation, and impaired healing phenomena, collectively leading to carcinogenesis in diabetic conditions. Diabetes-associated epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition (EndMT) contribute to cancer-associated fibroblast (CAF) formation in tumors, allowing the epithelium and endothelium to enable tumor cell extravasation. In this review, we discuss the risk of cancer associated with anti-diabetic therapies, including DPP-4 inhibitors and SGLT2 inhibitors, and the role of catechol-o-methyltransferase (COMT), AMPK, and cell-specific glucocorticoid receptors in cancer biology. We explore possible mechanistic links between diabetes and cancer biology and discuss new therapeutic approaches.
Keywords: AMPK activators; PPPM; antineoplastic therapy and diabetes; catechol-o-methyl-transferase; dipeptidyl peptidase 4; endothelial-cell glucocorticoid receptor; endothelial-to-mesenchymal transition; epithelial-to-mesenchymal transition; incretins; insulin; metformin; multiomics; sodium-glucose cotransporter 2; thiazolidinediones; type I diabetes mellitus and cancer and type II diabetes mellitus and cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

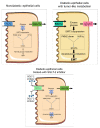
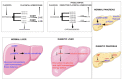
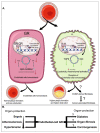
References
-
- Emerging Risk Factors C., Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

