Anti-PSMA CAR-engineered NK-92 Cells: An Off-the-shelf Cell Therapy for Prostate Cancer
- PMID: 32498368
- PMCID: PMC7349573
- DOI: 10.3390/cells9061382
Anti-PSMA CAR-engineered NK-92 Cells: An Off-the-shelf Cell Therapy for Prostate Cancer
Abstract
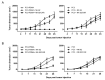
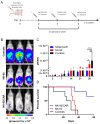
Prostate cancer (PCa) has become the most common cancer among males in Europe and the USA. Adoptive immunotherapy appears a promising strategy to control the advanced stages of the disease by specifically targeting the tumor, in particular through chimeric antigen receptor T (CAR-T) cell therapy. Despite the advancements of CAR-T technology in the treatment of hematological malignancies, solid tumors still represent a challenge. To overcome current limits, other cellular effectors than T lymphocytes are under study as possible candidates for CAR-engineered cancer immunotherapy. A novel approach involves the NK-92 cell line, which mediates strong cytotoxic responses against a variety of tumor cells but has no effect on non-malignant healthy counterparts. Here, we report a novel therapeutic approach against PCa based on engineering of NK-92 cells with a CAR recognizing the human prostate-specific membrane antigen (PSMA), which is overexpressed in prostatic neoplastic cells. More importantly, the potential utility of NK-92/CAR cells to treat PCa has not yet been explored. Upon CAR transduction, NK-92/CAR cells acquired high and specific lytic activity against PSMA-expressing prostate cancer cells in vitro, and also underwent degranulation and produced high levels of IFN-γ in response to antigen recognition. Lethal irradiation of the effectors, a safety measure requested for the clinical application of retargeted NK-92 cells, fully abrogated replication but did not impact on phenotype and short-term functionality. PSMA-specific recognition and antitumor activity were retained in vivo, as adoptive transfer of irradiated NK-92/CAR cells in prostate cancer-bearing mice restrained tumor growth and improved survival. Anti-PSMA CAR-modified NK-92 cells represent a universal, off-the-shelf, renewable, and cost-effective product endowed with relevant potentialities as a therapeutic approach for PCa immunotherapy.
Keywords: CAR; NK-92 cell line; PSMA; cancer immunotherapy; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells.J Immunother Cancer. 2018 Dec 4;6(1):136. doi: 10.1186/s40425-018-0441-8. J Immunother Cancer. 2018. PMID: 30514403 Free PMC article.
-
Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients.Mol Ther. 2019 Jun 5;27(6):1114-1125. doi: 10.1016/j.ymthe.2019.03.011. Epub 2019 Mar 20. Mol Ther. 2019. PMID: 30962163 Free PMC article.
-
Irradiated chimeric antigen receptor engineered NK-92MI cells show effective cytotoxicity against CD19+ malignancy in a mouse model.Cytotherapy. 2020 Oct;22(10):552-562. doi: 10.1016/j.jcyt.2020.06.003. Epub 2020 Aug 1. Cytotherapy. 2020. PMID: 32747298
-
CAR-NK Cells: From Natural Basis to Design for Kill.Front Immunol. 2021 Dec 14;12:707542. doi: 10.3389/fimmu.2021.707542. eCollection 2021. Front Immunol. 2021. PMID: 34970253 Free PMC article. Review.
-
CAR-NK cell in cancer immunotherapy; A promising frontier.Cancer Sci. 2021 Sep;112(9):3427-3436. doi: 10.1111/cas.14993. Epub 2021 Jul 7. Cancer Sci. 2021. PMID: 34050690 Free PMC article. Review.
Cited by
-
Allogeneic Expanded Human Peripheral NK Cells Control Prostate Cancer Growth in a Preclinical Mouse Model of Castration-Resistant Prostate Cancer.J Immunol Res. 2022 Apr 11;2022:1786395. doi: 10.1155/2022/1786395. eCollection 2022. J Immunol Res. 2022. PMID: 35450395 Free PMC article.
-
Bispecific PSMA antibodies and CAR-T in metastatic castration-resistant prostate cancer.Ther Adv Urol. 2023 Jun 15;15:17562872231182219. doi: 10.1177/17562872231182219. eCollection 2023 Jan-Dec. Ther Adv Urol. 2023. PMID: 37359737 Free PMC article. Review.
-
Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies.Stem Cell Res Ther. 2022 Sep 24;13(1):482. doi: 10.1186/s13287-022-03163-w. Stem Cell Res Ther. 2022. PMID: 36153626 Free PMC article. Review.
-
Arming Immune Cells for Battle: A Brief Journey through the Advancements of T and NK Cell Immunotherapy.Cancers (Basel). 2021 Mar 23;13(6):1481. doi: 10.3390/cancers13061481. Cancers (Basel). 2021. PMID: 33807011 Free PMC article. Review.
-
The Next Generation of Cellular Immunotherapy: Chimeric Antigen Receptor-Natural Killer Cells.Transplant Cell Ther. 2022 Oct;28(10):650-656. doi: 10.1016/j.jtct.2022.06.025. Epub 2022 Jul 3. Transplant Cell Ther. 2022. PMID: 35788086 Free PMC article. Review.
References
-
- Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M., et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. - DOI - PMC - PubMed
-
- Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

