Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression
- PMID: 32498400
- PMCID: PMC7312363
- DOI: 10.3390/ijms21113990
Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression
Abstract
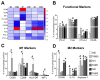
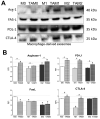
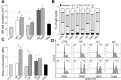
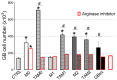
Interactions between tumor cells and tumor-associated macrophages (TAMs) are critical for glioblastoma progression. The TAMs represent up to 30% of the glioblastoma mass. The role of TAMs in tumor progression and in the mechanisms underlying tumor growth remain unclear. Using an in vitro model resembling the crosstalk between macrophages and glioblastoma cells, we show that glioblastoma-derived exosomes (GBex) reprogram M1 (mediate pro-inflammatory function) and M2 (mediate anti-inflammatory function) macrophages, converting M1 into TAMs and augmenting pro-tumor functions of M2 macrophages. In turn, these GBex-reprogrammed TAMs, produce exosomes decorated by immunosuppressive and tumor-growth promoting proteins. TAM-derived exosomes disseminate these proteins in the tumor microenvironment (TME) promoting tumor cell migration and proliferation. Mechanisms underlying the promotion of glioblastoma growth involved Arginase-1+ exosomes produced by the reprogrammed TAMs. A selective Arginase-1 inhibitor, nor-NOHA reversed growth-promoting effects of Arginase-1 carried by TAM-derived exosomes. The data suggest that GBex-reprogrammed Arginase-1+ TAMs emerge as a major source of exosomes promoting tumor growth and as a potential therapeutic target in glioblastoma.
Keywords: Arginase-1; TAM-derived exosomes; glioblastoma; glioblastoma--derived exosomes (GBex); macrophage reprogramming; tumor-associated macrophages (TAMs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

