Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy
- PMID: 32498474
- PMCID: PMC7348939
- DOI: 10.3390/cells9061385
Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy
Abstract
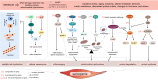
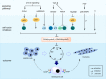
Sarcopenia has been defined as a progressive decline of skeletal muscle mass, strength, and functions in elderly people. It is accompanied by physical frailty, functional disability, falls, hospitalization, and mortality, and is becoming a major geriatric disorder owing to the increasing life expectancy and growing older population worldwide. Experimental models are critical to understand the pathophysiology of sarcopenia and develop therapeutic strategies. Although its etiologies remain to be further elucidated, several mechanisms of sarcopenia have been identified, including cellular senescence, proteostasis imbalance, oxidative stress, and "inflammaging." In this article, we address three main aspects. First, we describe the fundamental aging mechanisms. Next, we discuss both in vitro and in vivo experimental models based on molecular mechanisms that have the potential to elucidate the biochemical processes integral to sarcopenia. The use of appropriate models to reflect sarcopenia and/or its underlying pathways will enable researchers to understand sarcopenia and develop novel therapeutic strategies for sarcopenia. Lastly, we discuss the possible molecular targets and the current status of drug candidates for sarcopenia treatment. In conclusion, the development of experimental models for sarcopenia is essential to discover molecular targets that are valuable as biochemical biomarkers and/or therapeutic targets for sarcopenia.
Keywords: aging; cellular senescence; experimental model; sarcopenia; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

