Psychotherapy or medication for depression? Using individual symptom meta-analyses to derive a Symptom-Oriented Therapy (SOrT) metric for a personalised psychiatry
- PMID: 32498707
- PMCID: PMC7273646
- DOI: 10.1186/s12916-020-01623-9
Psychotherapy or medication for depression? Using individual symptom meta-analyses to derive a Symptom-Oriented Therapy (SOrT) metric for a personalised psychiatry
Abstract
Background: Antidepressant medication (ADM) and psychotherapy are effective treatments for major depressive disorder (MDD). It is unclear, however, if treatments differ in their effectiveness at the symptom level and whether symptom information can be utilised to inform treatment allocation. The present study synthesises comparative effectiveness information from randomised controlled trials (RCTs) of ADM versus psychotherapy for MDD at the symptom level and develops and tests the Symptom-Oriented Therapy (SOrT) metric for precision treatment allocation.
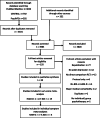
Methods: First, we conducted systematic review and meta-analyses of RCTs comparing ADM and psychotherapy at the individual symptom level. We searched PubMed Medline, PsycINFO, and the Cochrane Central Register of Controlled Trials databases, a database specific for psychotherapy RCTs, and looked for unpublished RCTs. Random-effects meta-analyses were applied on sum-scores and for individual symptoms for the Hamilton Rating Scale for Depression (HAM-D) and Beck Depression Inventory (BDI) measures. Second, we computed the SOrT metric, which combines meta-analytic effect sizes with patients' symptom profiles. The SOrT metric was evaluated using data from the Munich Antidepressant Response Signature (MARS) study (n = 407) and the Emory Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study (n = 234).
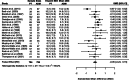
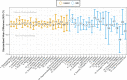
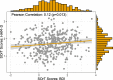
Results: The systematic review identified 38 RCTs for qualitative inclusion, 27 and 19 for quantitative inclusion at the sum-score level, and 9 and 4 for quantitative inclusion on individual symptom level for the HAM-D and BDI, respectively. Neither meta-analytic strategy revealed significant differences in the effectiveness of ADM and psychotherapy across the two depression measures. The SOrT metric did not show meaningful associations with other clinical variables in the MARS sample, and there was no indication of utility of the metric for better treatment allocation from PReDICT data.
Conclusions: This registered report showed no differences of ADM and psychotherapy for the treatment of MDD at sum-score and symptom levels. Symptom-based metrics such as the proposed SOrT metric do not inform allocation to these treatments, but predictive value of symptom information requires further testing for other treatment comparisons.
Keywords: Antidepressant medication; Depression symptoms; Major depressive disorder; Meta-analysis; Precision psychiatry; Psychotherapy; Symptom-oriented therapy metric; Systematic review.
Conflict of interest statement
NK, MR, JF, BAA, AGB, MM, GP, AGB, JD, JP, and JKB do not have any competing financial or other interests relating to the content of this study. HSM holds intellectual property in the field of deep brain stimulation for depression and is a consultant to Abbott Labs who has licenced the IP. WEC is a board member of Hugarheill ehf, an Icelandic company dedicated to the prevention of depression, and he receives book royalties from John Wiley & Sons. His research is supported by the NIH, the Mary and John Brock Foundation, and the Fuqua family foundations. He is a consultant to the George West Mental Health Foundation and is a member of the Scientific Advisory Boards of the ADAA and AIM for Mental Health. BWD has received research support from Acadia, Assurex Health, Axsome, Intra-Cellular Therapies, Janssen, National Institute of Mental Health, and Takeda. He has served as a consultant to Assurex Health and Aptinyx. CBN has received funding from the National Institutes of Health and the Stanley Medical Research Institute. In the last 3 years, he has served as a consultant to Xhale, Takeda, Taisho Pharmaceutical Inc., Signant Health, Sunovion Pharmaceuticals Inc., Janssen Research & Development LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., TC MSO, Inc., Intra-Cellular Therapies, Inc., EMA Wellness, Gerson Lehrman Group (GLG), and Acadia Pharmaceuticals, and served on the Board of Directors for the Gratitude America, Anxiety Disorders Association of America (ADAA), and Xhale Smart, Inc. CBN is a stockholder in Xhale, Celgene, Seattle Genetics, Abbvie, OPKO Health, Inc., Antares, BI Gen Holdings, Inc., Corcept Therapeutics Pharmaceuticals Company, TC MSO, Inc., Trends in Pharma Development, LLC, and EMA Wellness, and serves on the Scientific Advisory Boards of the American Foundation for Suicide Prevention (AFSP), Brain and Behavior Research Foundation (BBRF), Xhale, ADAA, Skyland Trail, Signant Health, Laureate Institute for Brain Research (LIBR), Inc. CBN reports income sources or equity of $10,000 or more from American Psychiatric Publishing, Xhale, Signant Health, CME Outfitters, Intra-Cellular Therapies, Inc., Magstim, and EMA Wellness, and has patents on the method and devices for transdermal delivery of lithium (US 6,375,990B1), the method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2), and Compounds, Compositions, Methods of Synthesis, and Methods of Treatment (CRF Receptor Binding Ligand) (US 8,551, 996 B2). MK is supported by the NIH and The John J. McDonnell and Margaret T.O. O’Brien Foundation. DNK receives grant funding from the National Institute of Mental Health (NIMH). NH is the chair of the board of trustees of Manchester Global Foundation (MGF) which was founded in 2015 as a Charitable Incorporated Organisation (CIO) registered in England and Wales. NH is the past Trustee of Pakistan Institute of Living & Learning (PILL), Abaseen Foundation and Lancashire Mind. NH has attended educational events sponsored by pharmaceutical industry. RBJ’s medical center collects the payments from the cognitive therapy she provides to patients. RBJ is a paid consultant to the National Institute of Mental Health and is a paid reviewer for UpToDate. She owns stock equity in Amgen, Johnson and Johnson, and Procter and Gamble. JRV is a paid reviewer for UpToDate. JPB received medication and placebo from Pfizer and NIMH funding for the data he contributed to the present study. MEK reports the following potential conflicts of interest: speakers bureau honoraria and other continuing medical education activity: AstraZeneca (Switzerland), Eli Lilly (Switzerland), Lundbeck (Switzerland), Vifor (Switzerland), and Zeller (Switzerland), as well as advisory panel payment from Lundbeck Switzerland.
Figures


References
-
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJM, et al. The FKBP5-gene in depression and treatment response—an association study in the sequenced treatment alternatives to relieve depression (STAR*D) cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 MH077869/MH/NIMH NIH HHS/United States
- M01 RR0039/Foundation for the National Institutes of Health/International
- 01ES0811/Bundesministerium für Bildung und Forschung (DE)/International
- P50 MH077083/MH/NIMH NIH HHS/United States
- R01 MH073719/MH/NIMH NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- T32 GM008695/GM/NIGMS NIH HHS/United States
- R01 MH080880/MH/NIMH NIH HHS/United States
- R01 MH061410/MH/NIMH NIH HHS/United States
- K23 MH086690/MH/NIMH NIH HHS/United States
- 01EE1401D/Bundesministerium für Bildung und Forschung/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

