Lenalidomide before and after autologous stem cell transplantation for transplant-eligible patients of all ages in the randomized, phase III, Myeloma XI trial
- PMID: 32499244
- PMCID: PMC8252959
- DOI: 10.3324/haematol.2020.247130
Lenalidomide before and after autologous stem cell transplantation for transplant-eligible patients of all ages in the randomized, phase III, Myeloma XI trial
Abstract
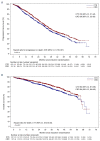
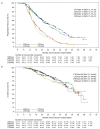
The optimal way to use immunomodulatory drugs as components of induction and maintenance therapy for multiple myeloma is unresolved. We addressed this question in a large phase III randomized trial, Myeloma XI. Patients with newly diagnosed multiple myeloma (n = 2042) were randomized to induction therapy with cyclophosphamide, thalidomide, and dexamethasone (CTD) or cyclophosphamide, lenalidomide, and dexamethasone (CRD). Additional intensification therapy with cyclophosphamide, bortezomib and dexamethasone (CVD) was administered before ASCT to patients with a suboptimal response to induction therapy using a response-adapted approach. After receiving high-dose melphalan with autologous stem cell transplantation (ASCT), eligible patients were further randomized to receive either lenalidomide alone or observation alone. Co-primary endpoints were progression-free survival (PFS) and overall survival (OS). The CRD regimen was associated with significantly longer PFS (median: 36 vs. 33 months; hazard ratio [HR], 0.85; 95% confidence interval [CI], 0.75-0.96; P = 0.0116) and OS (3-year OS: 82.9% vs. 77.0%; HR, 0.77; 95% CI, 0.63-0.93; P = 0.0072) compared with CTD. The PFS and OS results favored CRD over CTD across all subgroups, including patients with International Staging System stage III disease (HR for PFS, 0.73; 95% CI, 0.58-0.93; HR for OS, 0.78; 95% CI, 0.56-1.09), high-risk cytogenetics (HR for PFS, 0.60; 95% CI, 0.43-0.84; HR for OS, 0.70; 95% CI, 0.42-1.15) and ultra high-risk cytogenetics (HR for PFS, 0.67; 95% CI, 0.41-1.11; HR for OS, 0.65; 95% CI, 0.34-1.25). Among patients randomized to lenalidomide maintenance (n = 451) or observation (n = 377), maintenance therapy improved PFS (median: 50 vs. 28 months; HR, 0.47; 95% CI, 0.37-0.60; P < 0.0001). Optimal results for PFS and OS were achieved in the patients who received CRD induction and lenalidomide maintenance. The trial was registered with the EU Clinical Trials Register (EudraCT 2009-010956-93) and ISRCTN49407852.
Figures

References
-
- Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28(6):1346-1348. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. . High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875-1883. - PubMed
-
- Moreau P, Avet-Loiseau H, Facon T, et al. . Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752-5758. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

