Monitoring patients with acute dyspnoea with a serial focused ultrasound of the heart and the lungs (MODUS): a protocol for a multicentre, randomised, open-label, pragmatic and controlled trial
- PMID: 32499263
- PMCID: PMC7279664
- DOI: 10.1136/bmjopen-2019-034373
Monitoring patients with acute dyspnoea with a serial focused ultrasound of the heart and the lungs (MODUS): a protocol for a multicentre, randomised, open-label, pragmatic and controlled trial
Abstract
Introduction: Among patients admitted to an emergency department, dyspnoea is one of the most common symptoms. Patients with dyspnoea have high mortality and morbidity. Therefore, novel methods to monitor the patients are warranted. The aim is to investigate whether therapy guided by monitoring patients with acute dyspnoea with serial ultrasound examinations of the heart and the lungs together with standard care can change the severity of dyspnoea compared with treatment guided by standard monitoring alone.
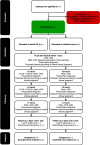
Methods and analysis: The study will be conducted as a multicentre, randomised, pragmatic, open-label and controlled trial where patients admitted with acute dyspnoea to an emergency ward will be randomised into a standard care group and a serial ultrasound group with 103 patients in each. All patients will be examined with an ultrasound of the heart and the lungs upfront. In addition, the patients in the serial ultrasound group will be examined with an ultrasound of the heart and lungs two more times to guide further therapy during the admittance. The primary outcome is a change in dyspnoea on a verbal scale. After discharge, the patients are followed for 1 year to assess the number of readmissions, death and length of hospital stay.
Ethics and dissemination: The trial is conducted in accordance with the Declaration of Helsinki and approved by The Regional Committee on Health Research Ethics for Region Zealand, Denmark (identifier SJ-744). Data handling agreement with participating centres has been made (identifier REG-056-2019). The General Data Protection Regulation and the Danish Data Protection Act will be respected. The results of the trial will be reported in peer-reviewed scientific journals regardless of the outcomes.
Trial registration number: NCT04091334.
Keywords: dyspnoea; focused cardiac ultrasound; inferior vena cava; lung ultrasound; monitoring; serial.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
