Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn's disease: a multicentre, prospective study
- PMID: 32499275
- PMCID: PMC7282309
- DOI: 10.1136/bmjgast-2019-000365
Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn's disease: a multicentre, prospective study
Abstract
Introduction: Crohn's disease diagnosis and monitoring remains a great clinical challenge and often requires multiple testing modalities. Assessing Crohn's disease activity in the entire gastrointestinal (GI) tract using a panenteric capsule endoscopy (CE) system could be used as an alternative to colonoscopy and cross-sectional imaging. This study assessed the accuracy and safety of panenteric CE in Crohn's disease as compared with ileocolonoscopy (IC) and/or magnetic resonance enterography (MRE).
Methods: A prospective, multicentre study was performed in subjects with established Crohn's disease. Individuals with proven small bowel patency underwent a standardised bowel preparation, followed by CE ingestion and IC either the same or following day. MRE, IC, and CE interpretations were performed by blinded central readers using validated scoring systems. The primary endpoint was the overall sensitivity of CE vs MRE and/or IC in Crohn's disease subjects.
Results: Study enrolment included 158 subjects from 21 sites in the USA, Austria, and Israel. Of those, 99 were included in the analysis. Imaging modality scores indicated none to mild inflammation in the proximal small bowel and colon, but discrepant levels of inflammation in the terminal ileum. Overall sensitivity for active enteric inflammation (CE vs MRE and/or IC) was 94% vs 100% (p=0.125) and specificity was 74% vs 22% (p=0.001). Sensitivity of CE was superior to MRE for enteric inflammation in the proximal small bowel (97% vs 71%, p=0.021), and similar to MRE and/or IC in the terminal ileum and colon (p=0.500-0.625). There were seven serious adverse advents of which three were related to the CE device.
Conclusion: Panenteric CE is a reliable tool for assessing Crohn's disease mucosal activity and extent compared with more invasive methods. This study demonstrates high performance of the panenteric CE as compared to MRE and/or IC without the need for multiple tests in non-stricturing Crohn's disease.
Trial registration number: ClinicalTrials.gov NCT03241368.
Keywords: Crohn's disease; colonoscopy; endoscopy; inflammatory bowel disease; small bowel disease.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DHB declares consulting for Medtronic. SO declares research funding from Medtronic.
Figures
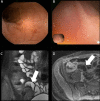
Similar articles
-
Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn's disease.Clin Gastroenterol Hepatol. 2014 Apr;12(4):609-15. doi: 10.1016/j.cgh.2013.09.028. Epub 2013 Sep 27. Clin Gastroenterol Hepatol. 2014. PMID: 24075891
-
Small-bowel imaging in Crohn's disease: a prospective, blinded, 4-way comparison trial.Gastrointest Endosc. 2008 Aug;68(2):255-66. doi: 10.1016/j.gie.2008.02.017. Epub 2008 Jun 2. Gastrointest Endosc. 2008. PMID: 18513722 Clinical Trial.
-
Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric Crohn's disease: a prospective, blinded, comparison study.Gastrointest Endosc. 2015 Feb;81(2):420-7. doi: 10.1016/j.gie.2014.07.009. Epub 2014 Aug 10. Gastrointest Endosc. 2015. PMID: 25115363 Clinical Trial.
-
Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn's disease: Systematic review and meta-analysis.Dig Liver Dis. 2017 Aug;49(8):854-863. doi: 10.1016/j.dld.2017.04.013. Epub 2017 Apr 27. Dig Liver Dis. 2017. PMID: 28512034
-
Capsule Endoscopy, Magnetic Resonance Enterography, and Small Bowel Ultrasound for Evaluation of Postoperative Recurrence in Crohn's Disease: Systematic Review and Meta-Analysis.Inflamm Bowel Dis. 2017 Dec 19;24(1):93-100. doi: 10.1093/ibd/izx027. Inflamm Bowel Dis. 2017. PMID: 29272490
Cited by
-
Usefulness of Serum Leucine-Rich Alpha-2 Glycoprotein as a Surrogate Marker of Small Bowel Mucosal Injury in Crohn's Disease.Inflamm Intest Dis. 2023 Jul 10;8(2):69-76. doi: 10.1159/000531622. eCollection 2023 Oct. Inflamm Intest Dis. 2023. PMID: 37901342 Free PMC article.
-
The role of small-bowel endoscopy in inflammatory bowel disease: an updated review on the state-of-the-art in 2021.Ann Gastroenterol. 2021 Sep-Oct;34(5):599-611. doi: 10.20524/aog.2021.0652. Epub 2021 Jul 2. Ann Gastroenterol. 2021. PMID: 34475730 Free PMC article. Review.
-
Capsule endoscopy and panendoscopy: A journey to the future of gastrointestinal endoscopy.World J Gastroenterol. 2024 Mar 14;30(10):1270-1279. doi: 10.3748/wjg.v30.i10.1270. World J Gastroenterol. 2024. PMID: 38596501 Free PMC article.
-
Capsule Endoscopy in Inflammatory Bowel Disease: Panenteric Capsule Endoscopy and Application of Artificial Intelligence.Gut Liver. 2023 Jul 15;17(4):516-528. doi: 10.5009/gnl220507. Epub 2023 Jun 12. Gut Liver. 2023. PMID: 37305947 Free PMC article.
-
Utility of panenteric capsule endoscopy for the detection of small-bowel Crohn's disease in patients with a normal magnetic resonance enterography: A prospective observational pilot study.JGH Open. 2023 Nov 27;7(12):966-973. doi: 10.1002/jgh3.13013. eCollection 2023 Dec. JGH Open. 2023. PMID: 38162838 Free PMC article.
References
-
- Cellier C, Sahmoud T, Froguel E, et al. . Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994;35:231–5. 10.1136/gut.35.2.231 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
