Impact of Microbiome on Hepatic Metabolizing Enzymes and Transporters in Mice during Pregnancy
- PMID: 32499338
- PMCID: PMC7434050
- DOI: 10.1124/dmd.120.000039
Impact of Microbiome on Hepatic Metabolizing Enzymes and Transporters in Mice during Pregnancy
Abstract
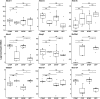
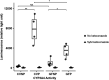
The microbiome and pregnancy are known to alter drug disposition, yet the interplay of the two physiologic factors on the expression and/or activity of drug metabolizing enzymes and transporters (DMETs) is unknown. This study investigated the effects of microbiome on host hepatic DMETs in mice during pregnancy by comparing four groups of conventional (CV) and germ-free (GF) female mice and pregnancy status, namely, CV nonpregnant, GF non-pregnant, CV pregnant, and GF pregnant mice. Transcriptomic and targeted proteomics of hepatic DMETs were profiled by using multiomics. Plasma bile acid and steroid hormone levels were quantified by liquid chromatography tandem mass spectrometry. CYP3A activities were measured by mouse liver microsome incubations. The trend of pregnancy-induced changes in the expression or activity of hepatic DMETs in CV and GF mice was similar; however, the magnitude of change was noticeably different. For certain DMETs, pregnancy status had paradoxical effects on mRNA and protein expression in both CV and GF mice. For instance, the mRNA levels of Cyp3a11, the murine homolog of human CYP3A4, were decreased by 1.7-fold and 3.3-fold by pregnancy in CV and GF mice, respectively; however, the protein levels of CYP3A11 were increased similarly ∼twofold by pregnancy in both CV and GF mice. Microsome incubations revealed a marked induction of CYP3A activity by pregnancy that was 10-fold greater in CV mice than that in GF mice. This is the first study to show that the microbiome can alter the expression and/or activity of hepatic DMETs in pregnancy. SIGNIFICANCE STATEMENT: We demonstrated for the first time that microbiome and pregnancy can interplay to alter the expression and/or activity of hepatic drug metabolizing enzymes and transporters. Though the trend of pregnancy-induced changes in the expression or activity of hepatic drug metabolizing enzymes and transporters in conventional and germ-free mice was similar, the magnitude of change was noticeably different.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures








References
-
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

