Role of the lipid bilayer in outer membrane protein folding in Gram-negative bacteria
- PMID: 32499369
- PMCID: PMC7383365
- DOI: 10.1074/jbc.REV120.011473
Role of the lipid bilayer in outer membrane protein folding in Gram-negative bacteria
Abstract
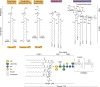
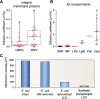
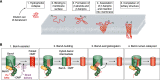
β-Barrel outer membrane proteins (OMPs) represent the major proteinaceous component of the outer membrane (OM) of Gram-negative bacteria. These proteins perform key roles in cell structure and morphology, nutrient acquisition, colonization and invasion, and protection against external toxic threats such as antibiotics. To become functional, OMPs must fold and insert into a crowded and asymmetric OM that lacks much freely accessible lipid. This feat is accomplished in the absence of an external energy source and is thought to be driven by the high thermodynamic stability of folded OMPs in the OM. With such a stable fold, the challenge that bacteria face in assembling OMPs into the OM is how to overcome the initial energy barrier of membrane insertion. In this review, we highlight the roles of the lipid environment and the OM in modulating the OMP-folding landscape and discuss the factors that guide folding in vitro and in vivo We particularly focus on the composition, architecture, and physical properties of the OM and how an understanding of the folding properties of OMPs in vitro can help explain the challenges they encounter during folding in vivo Current models of OMP biogenesis in the cellular environment are still in flux, but the stakes for improving the accuracy of these models are high. OMP folding is an essential process in all Gram-negative bacteria, and considering the looming crisis of widespread microbial drug resistance it is an attractive target. To bring down this vital OMP-supported barrier to antibiotics, we must first understand how bacterial cells build it.
Keywords: BAM complex; Gram-negative bacteria; OMPome; antibiotic resistance; disorderase; folding kinetics; lipid; lipid membrane; membrane bilayer; membrane protein; membrane protein folding; outer membrane; protein folding; protein-lipid interactions; β-barrel assembly machinery (BAM) complex.
© 2020 Horne et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BB/M01151/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K000659/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/T000635/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/N007603/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/P018491/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

