Enduring Changes in Neuronal Function upon Systemic Inflammation Are NLRP3 Inflammasome Dependent
- PMID: 32499379
- PMCID: PMC7343321
- DOI: 10.1523/JNEUROSCI.0200-20.2020
Enduring Changes in Neuronal Function upon Systemic Inflammation Are NLRP3 Inflammasome Dependent
Abstract
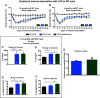
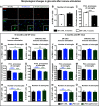
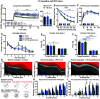
Neuroinflammation can be caused by various insults to the brain and represents an important pathologic hallmark of neurodegenerative diseases including Alzheimer's disease (AD). Infection-triggered acute systemic inflammation is able to induce neuroinflammation and may negatively affect neuronal morphology, synaptic plasticity, and cognitive function. In contrast to acute effects, persisting consequences for the brain on systemic immune stimulation remain largely unexplored. Here, we report an age-dependent vulnerability of wild-type (WT) mice of either sex toward a systemic immune stimulation by Salmonella typhimurium lipopolysaccharide (LPS). Decreased neuronal complexity three months after peripheral immune stimulation is accompanied by impairment in long-term potentiation (LTP) and spatial learning. Aged APP/PS1 mice reveal an increased sensitivity also to LPS of Escherichia coli, which had no effect in WT mice. We further report that these effects are mediated by NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome activation, since the genetic ablation and pharmacological inhibition using the NLRP3 inhibitor MCC950 rescue the morphological and electrophysiological phenotype.SIGNIFICANCE STATEMENT Acute peripheral immune stimulation has been shown to have both positive and negative effects on Aβ deposition. Improvements or worsening may be possible in acute inflammation. However, there is still no evidence of effects longer than a month after stimulation. The data are pointing to an important role of the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome for mediating the long-term consequences of systemic immune stimulation, which in addition turns out to be age dependent.
Keywords: APP/PS1; LPS; NLRP3; hippocampus; neuroinflammation; sepsis.
Copyright © 2020 the authors.
Figures




References
-
- Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, Kuijlaars J, Langlois X, Matthews LJR, Ver Donck L, Hellings N, Nuydens R (2013) Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm 2013:271359. 10.1155/2013/271359 - DOI - PMC - PubMed
-
- Bone RC. (1992) Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 268:3452–3455. - PubMed
-
- Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS (1997) Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19:939–945. 10.1016/S0896-6273(00)80974-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
