Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field
- PMID: 32499601
- PMCID: PMC7272635
- DOI: 10.1038/s41598-020-65830-3
Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field
Abstract
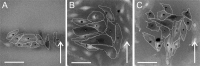
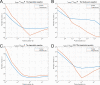
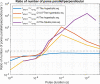
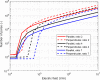
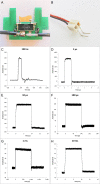
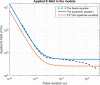
In gene electrotransfer and cardiac ablation with irreversible electroporation, treated muscle cells are typically of elongated shape and their orientation may vary. Orientation of cells in electric field has been reported to affect electroporation, and hence electrodes placement and pulse parameters choice in treatments for achieving homogeneous effect in tissue is important. We investigated how cell orientation influences electroporation with respect to different pulse durations (ns to ms range), both experimentally and numerically. Experimentally detected electroporation (evaluated separately for cells parallel and perpendicular to electric field) via Ca2+ uptake in H9c2 and AC16 cardiomyocytes was numerically modeled using the asymptotic pore equation. Results showed that cell orientation affects electroporation extent: using short, nanosecond pulses, cells perpendicular to electric field are significantly more electroporated than parallel (up to 100-times more pores formed), and with long, millisecond pulses, cells parallel to electric field are more electroporated than perpendicular (up to 1000-times more pores formed). In the range of a few microseconds, cells of both orientations were electroporated to the same extent. Using pulses of a few microseconds lends itself as a new possible strategy in achieving homogeneous electroporation in tissue with elongated cells of different orientation (e.g. electroporation-based cardiac ablation).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kotnik, T., Rems, L., Tarek, M. & Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 48, (2019). - PubMed
-
- Kinosita K, Tsong TY. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977;268:438–441. - PubMed
-
- Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014;16:295–320. - PubMed
-
- Marty M, et al. Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006;4:3–13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

