Feedback generates a second receptive field in neurons of the visual cortex
- PMID: 32499655
- PMCID: PMC7790439
- DOI: 10.1038/s41586-020-2319-4
Feedback generates a second receptive field in neurons of the visual cortex
Abstract
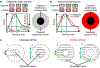
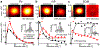
Animals sense the environment through pathways that link sensory organs to the brain. In the visual system, these feedforward pathways define the classical feedforward receptive field (ffRF), the area in space in which visual stimuli excite a neuron1. The visual system also uses visual context-the visual scene surrounding a stimulus-to predict the content of the stimulus2, and accordingly, neurons have been identified that are excited by stimuli outside their ffRF3-8. However, the mechanisms that generate excitation to stimuli outside the ffRF are unclear. Here we show that feedback projections onto excitatory neurons in the mouse primary visual cortex generate a second receptive field that is driven by stimuli outside the ffRF. The stimulation of this feedback receptive field (fbRF) elicits responses that are slower and are delayed in comparison with those resulting from the stimulation of the ffRF. These responses are preferentially reduced by anaesthesia and by silencing higher visual areas. Feedback inputs from higher visual areas have scattered receptive fields relative to their putative targets in the primary visual cortex, which enables the generation of the fbRF. Neurons with fbRFs are located in cortical layers that receive strong feedback projections and are absent in the main input layer, which is consistent with a laminar processing hierarchy. The observation that large, uniform stimuli-which cover both the fbRF and the ffRF-suppress these responses indicates that the fbRF and the ffRF are mutually antagonistic. Whereas somatostatin-expressing inhibitory neurons are driven by these large stimuli, inhibitory neurons that express parvalbumin and vasoactive intestinal peptide have mutually antagonistic fbRF and ffRF, similar to excitatory neurons. Feedback projections may therefore enable neurons to use context to estimate information that is missing from the ffRF and to report differences in stimulus features across visual space, regardless of whether excitation occurs inside or outside the ffRF. By complementing the ffRF, the fbRF that we identify here could contribute to predictive processing.
Conflict of interest statement
The authors declare no competing interests.
Figures
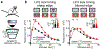

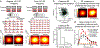









Similar articles
-
Columnar processing of border ownership in primate visual cortex.Elife. 2021 Nov 30;10:e72573. doi: 10.7554/eLife.72573. Elife. 2021. PMID: 34845986 Free PMC article.
-
Layer 3 Dynamically Coordinates Columnar Activity According to Spatial Context.J Neurosci. 2019 Jan 9;39(2):281-294. doi: 10.1523/JNEUROSCI.1568-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459226 Free PMC article.
-
Circuits and Mechanisms for Surround Modulation in Visual Cortex.Annu Rev Neurosci. 2017 Jul 25;40:425-451. doi: 10.1146/annurev-neuro-072116-031418. Epub 2017 May 3. Annu Rev Neurosci. 2017. PMID: 28471714 Free PMC article. Review.
-
Silencing "Top-Down" Cortical Signals Affects Spike-Responses of Neurons in Cat's "Intermediate" Visual Cortex.Front Neural Circuits. 2017 Apr 25;11:27. doi: 10.3389/fncir.2017.00027. eCollection 2017. Front Neural Circuits. 2017. PMID: 28487637 Free PMC article.
-
The contribution of vertical and horizontal connections to the receptive field center and surround in V1.Neural Netw. 2004 Jun-Jul;17(5-6):681-93. doi: 10.1016/j.neunet.2004.05.002. Neural Netw. 2004. PMID: 15288892 Review.
Cited by
-
Fast updating feedback from piriform cortex to the olfactory bulb relays multimodal reward contingency signals during rule-reversal.bioRxiv [Preprint]. 2023 Sep 13:2023.09.12.557267. doi: 10.1101/2023.09.12.557267. bioRxiv. 2023. Update in: Nat Commun. 2025 Jan 22;16(1):937. doi: 10.1038/s41467-025-56023-5. PMID: 37745564 Free PMC article. Updated. Preprint.
-
Dissection of brain-wide resting-state and functional somatosensory circuits by fMRI with optogenetic silencing.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2113313119. doi: 10.1073/pnas.2113313119. Proc Natl Acad Sci U S A. 2022. PMID: 35042795 Free PMC article.
-
Mouse Higher Visual Areas Provide Both Distributed and Specialized Contributions to Visually Guided Behaviors.Curr Biol. 2020 Dec 7;30(23):4682-4692.e7. doi: 10.1016/j.cub.2020.09.015. Epub 2020 Oct 8. Curr Biol. 2020. PMID: 33035487 Free PMC article.
-
Locomotion-induced gain of visual responses cannot explain visuomotor mismatch responses in layer 2/3 of primary visual cortex.Cell Rep. 2023 Mar 28;42(3):112096. doi: 10.1016/j.celrep.2023.112096. Epub 2023 Feb 22. Cell Rep. 2023. PMID: 36821437 Free PMC article.
-
Organization of feedback projections to mouse primary visual cortex.iScience. 2021 Apr 17;24(5):102450. doi: 10.1016/j.isci.2021.102450. eCollection 2021 May 21. iScience. 2021. PMID: 34113813 Free PMC article.
References
-
- Von der Heydt R, Peterhans E & Baumgartner G Illusory Contours and Cortical Neuron Responses. Science 224, 1260–1262 (1984). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

