Analysis of Electrocatalytic Metal-Organic Frameworks
- PMID: 32499663
- PMCID: PMC7272229
- DOI: 10.1016/j.ccr.2019.213137
Analysis of Electrocatalytic Metal-Organic Frameworks
Abstract
The electrochemical analysis of molecular catalysts for the conversion of bulk feedstocks into energy-rich clean fuels has seen dramatic advances in the last decade. More recently, increased attention has focused on the characterization of metal-organic frameworks (MOFs) containing well-defined redox and catalytically active sites, with the overall goal to develop structurally stable materials that are industrially relevant for large-scale solar fuel syntheses. Successful electrochemical analysis of such materials draws heavily on well-established homogeneous techniques, yet the nature of solid materials presents additional challenges. In this tutorial-style review, we cover the basics of electrochemical analysis of electroactive MOFs, including considerations of bulk stability, methods of attaching MOFs to electrodes, interpreting fundamental electrochemical data, and finally electrocatalytic kinetic characterization. We conclude with a perspective of some of the prospects and challenges in the field of electrocatalytic MOFs.
Keywords: electroactive thin films; electrocatalysis; electrochemistry; metal-organic frameworks.
Figures




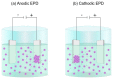





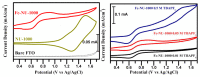
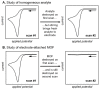




References
-
- U.S. Global Change Research Program. Fourth National Climate Assessment. 2018 doi: 10.1016/j.pbb.2008.09.016. - DOI
-
- Dziba L, Erpul G, Fazel A, Fischer M, Hernández AM. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Service. 2019
-
- McKone JR, Gray HB, Lewis NS. Will Solar-Driven Water-Splitting Devices See the Light of Day? Chen Mater. 2013 doi: 10.1021/cm4021518. - DOI
-
- Bard A, Fox MA. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc Chem Res. 1995;28:141–145. doi: 10.1021/ar00051a007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
