UVA Induced Oxidative Stress Was Inhibited by Paeoniflorin/Nrf2 Signaling or PLIN2
- PMID: 32499710
- PMCID: PMC7243259
- DOI: 10.3389/fphar.2020.00736
UVA Induced Oxidative Stress Was Inhibited by Paeoniflorin/Nrf2 Signaling or PLIN2
Abstract
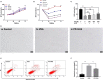
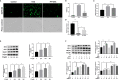
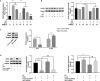
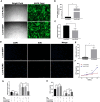
Photodamages caused by UVA radiation induced oxidative injuries are closely related to photoaging and skin cancer. Paeoniflorin (PF), extracted from the root of Paeonia lactiflora, has been reported to be an effective antioxidant. PLIN2, known as adipose differentiation-related protein, has been previously involved in the regulation of oxidative stress. In this study, we were sought to investigate the photo-protective property of PF and PLIN2 in UVA-radiated human dermal fibroblasts (HDFs). HDFs were pre-treated with PF (800 μM) followed by UVA radiation (22.5 J/cm2). MTS activity, cell apoptosis, ROS, MDA, and SOD were detected, respectively. The expressions of Nrf2, HO-1, NQ-O1, and PLIN2 were determined using RT-qPCR or western blot. Nrf2 was silenced by siRNA, and PLIN2 was overexpressed via lentiviral transduction. Comparing to the UVA radiation, PF pre-treatment could prominently increase the MTS activity, decrease cell apoptosis, reduce the generations of ROS and MDA, increase the activity of SOD and increase the expression of Nrf2 and its target genes HO-1 and NQ-O1. When Nrf2 was knocked down, PF lost above protective properties. In addition, UVA induced oxidative stress led to upregulation of PLIN2 and the latter could be decreased by PF. Overexpression of PLIN2 improved MTS activity and reduced MDA level in HDFs. The combination of PLIN2 overexpression and PF pre-treatment corporately inhibited UVA-induced injury. Besides, we also found that PF and PLIN2 had a compensatory protection against UVA induced oxidative stress. In conclusion, our study demonstrated that UVA induced photodamages could be inhibited by PF via Nrf2/HO-1/NQ-O1 signaling pathway or by PLIN2, and the combination of PLIN2 overexpression and PF played additive effects against UVA-related oxidative stress.
Keywords: Nrf2; PLIN2; UVA; oxidative stress; paeoniflorin.
Copyright © 2020 Lu, Jiang, Yuan, Jiang, Yang, Zhu, Sun, Qi, Liu, Wang, Wu, Gao and Chen.
Figures

Similar articles
-
Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis.J Ethnopharmacol. 2016 Jun 5;185:361-9. doi: 10.1016/j.jep.2016.03.031. Epub 2016 Mar 12. J Ethnopharmacol. 2016. PMID: 26979341
-
Pterostilbene's protective effects against photodamage caused by UVA/UVB irradiation.Pharmazie. 2018 Nov 1;73(11):651-658. doi: 10.1691/ph.2018.8598. Pharmazie. 2018. PMID: 30396384
-
Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes.Free Radic Biol Med. 2015 Sep;86:102-17. doi: 10.1016/j.freeradbiomed.2015.05.026. Epub 2015 May 27. Free Radic Biol Med. 2015. PMID: 26021820
-
Effects of low-dose ALA-PDT on fibroblast photoaging induced by UVA irradiation and the underlying mechanisms.Photodiagnosis Photodyn Ther. 2019 Sep;27:79-84. doi: 10.1016/j.pdpdt.2019.05.006. Epub 2019 May 7. Photodiagnosis Photodyn Ther. 2019. PMID: 31075320
-
Caffeic acid derivatives isolated from Galinsoga parviflora herb protected human dermal fibroblasts from UVA-radiation.Phytomedicine. 2019 Apr;57:215-222. doi: 10.1016/j.phymed.2018.12.022. Epub 2018 Dec 17. Phytomedicine. 2019. PMID: 30785017
Cited by
-
Total Glucosides of Peony Protect Cardiomyocytes against Oxidative Stress and Inflammation by Reversing Mitochondrial Dynamics and Bioenergetics.Oxid Med Cell Longev. 2020 Nov 25;2020:6632413. doi: 10.1155/2020/6632413. eCollection 2020. Oxid Med Cell Longev. 2020. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9826087. doi: 10.1155/2023/9826087. PMID: 33354278 Free PMC article. Retracted.
-
Screening of potential antioxidant bioactive Q-markers of paeoniae radix rubra based on an integrated multimodal strategy.Front Pharmacol. 2024 Aug 15;15:1447959. doi: 10.3389/fphar.2024.1447959. eCollection 2024. Front Pharmacol. 2024. PMID: 39211775 Free PMC article.
-
Inhibition of perilipin 2 attenuates cerebral ischemia/reperfusion injury by blocking NLRP3 inflammasome activation both in vivo and in vitro.In Vitro Cell Dev Biol Anim. 2023 Mar;59(3):204-213. doi: 10.1007/s11626-023-00759-1. Epub 2023 Apr 3. In Vitro Cell Dev Biol Anim. 2023. PMID: 37010675
-
Oleuropein-Rich Gellan Gum/Alginate Films as Innovative Treatments against Photo-Induced Skin Aging.Molecules. 2023 May 25;28(11):4352. doi: 10.3390/molecules28114352. Molecules. 2023. PMID: 37298828 Free PMC article.
-
Expression of AMPK and PLIN2 in the regulation of lipid metabolism and oxidative stress in bitches with open cervix pyometra.BMC Vet Res. 2025 Mar 13;21(1):164. doi: 10.1186/s12917-025-04622-1. BMC Vet Res. 2025. PMID: 40082867 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

