Differential Regulation of Kernel Set and Potential Kernel Weight by Nitrogen Supply and Carbohydrate Availability in Maize Genotypes Contrasting in Nitrogen Use Efficiency
- PMID: 32499807
- PMCID: PMC7243938
- DOI: 10.3389/fpls.2020.00586
Differential Regulation of Kernel Set and Potential Kernel Weight by Nitrogen Supply and Carbohydrate Availability in Maize Genotypes Contrasting in Nitrogen Use Efficiency
Abstract
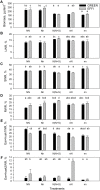
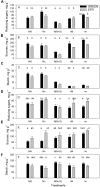
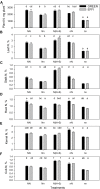
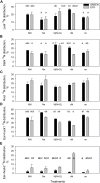
Sub-optimal nitrogen (N) conditions reduce maize yield due to a decrease in two sink components: kernel set and potential kernel weight. Both components are established during the lag phase, suggesting that they could compete for resources during this critical period. However, whether this competition occurs or whether different genotypic strategies exist to optimize photoassimilate use during the lag phase is not clear and requires further investigation. We have addressed this knowledge gap by conducting a nutrient solution culture experiment that allows abrupt changes in N level and light intensity during the lag phase. We investigated plant growth, dry matter partitioning, non-structural carbohydrate concentration, N concentration, and 15N distribution (applied 4 days before silking) in plant organs at the beginning and the end of the lag phase in two maize hybrids that differ in grain yield under N-limited conditions: one is a nitrogen-use-efficient (EFFI) genotype and the other is a control (GREEN) genotype that does not display high N use efficiency. We found that the two genotypes used different mechanisms to regulate kernel set. The GREEN genotype showed a reduction in kernel set associated with reduced dry matter allocation to the ear during the lag phase, indicating that the reduced kernel set under N-limited conditions was related to sink restrictions. This idea was supported by a negative correlation between kernel set and sucrose/total sugar ratios in the kernels, indicating that the capacity for sucrose cleavage might be a key factor defining kernel set in the GREEN genotype. By contrast, the kernel set of the EFFI genotype was not correlated with dry matter allocation to the ear or to a higher capacity for sucrose cleavage; rather, it showed a relationship with the different EFFI ear morphology with bigger kernels at the apex of the ear than in the GREEN genotype. The potential kernel weight was independent of carbohydrate availability but was related to the N flux per kernel in both genotypes. In conclusion, kernel set and potential kernel weight are regulated independently, suggesting the possibility of simultaneously increasing both sink components in maize.
Keywords: kernel set; maize; nitrogen; nitrogen use efficiency; non-structural carbohydrate; potential kernel weight; sink strength.
Copyright © 2020 Paponov, Paponov, Sambo and Engels.
Figures




Similar articles
-
Combating Dual Challenges in Maize Under High Planting Density: Stem Lodging and Kernel Abortion.Front Plant Sci. 2021 Nov 2;12:699085. doi: 10.3389/fpls.2021.699085. eCollection 2021. Front Plant Sci. 2021. PMID: 34868101 Free PMC article. Review.
-
Which plant traits are most strongly related to post-silking nitrogen uptake in maize under water and/or nitrogen stress?J Plant Physiol. 2020 Jan;244:153059. doi: 10.1016/j.jplph.2019.153059. Epub 2019 Nov 2. J Plant Physiol. 2020. PMID: 31775101
-
Interaction of carbon and nitrogen metabolism in the productivity of maize.Plant Physiol. 1982 Oct;70(4):1185-90. doi: 10.1104/pp.70.4.1185. Plant Physiol. 1982. PMID: 16662636 Free PMC article.
-
Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize.J Exp Bot. 2018 Mar 24;69(7):1707-1719. doi: 10.1093/jxb/erx496. J Exp Bot. 2018. PMID: 29361032 Free PMC article.
-
Maize reproductive development and kernel set under limited plant growth environments.J Exp Bot. 2018 Jun 6;69(13):3235-3243. doi: 10.1093/jxb/erx452. J Exp Bot. 2018. PMID: 29304259 Review.
Cited by
-
Effects of combined nitrogen and phosphorus application on protein fractions and nonstructural carbohydrate of alfalfa.Front Plant Sci. 2023 Mar 8;14:1124664. doi: 10.3389/fpls.2023.1124664. eCollection 2023. Front Plant Sci. 2023. PMID: 36968423 Free PMC article.
-
Combating Dual Challenges in Maize Under High Planting Density: Stem Lodging and Kernel Abortion.Front Plant Sci. 2021 Nov 2;12:699085. doi: 10.3389/fpls.2021.699085. eCollection 2021. Front Plant Sci. 2021. PMID: 34868101 Free PMC article. Review.
-
The Physiological Mechanisms and Hurdles of Efficient Water-Nitrogen Utilization in Maize Production: A Review.Plants (Basel). 2025 Jun 20;14(13):1899. doi: 10.3390/plants14131899. Plants (Basel). 2025. PMID: 40647908 Free PMC article. Review.
-
Adjusting sowing date improves the photosynthetic capacity and grain yield by optimizing temperature condition around flowering of summer maize in the North China Plain.Front Plant Sci. 2022 Aug 8;13:934618. doi: 10.3389/fpls.2022.934618. eCollection 2022. Front Plant Sci. 2022. PMID: 36003815 Free PMC article.
References
-
- Andrade F. H., Echarte L., Rizzalli R., Della Maggiora A., Casanovas M. (2002). Kernel number prediction in maize under nitrogen or water stress. Crop Sci. 42 1173–1179. 10.2135/cropsci2002.1173 - DOI
-
- Blakeney A. B., Mutton L. L. (1980). A simple colorimetric method for the determination of sugars in fruit and vegetables. J. Sci. Food Agr. 31 889–897. 10.1002/jsfa.2740310905 - DOI
-
- Borras L., Gambin B. L. (2010). Trait dissection of maize kernel weight: Towards integrating hierarchical scales using a plant growth approach. Field Crop. Res. 118 1–12. 10.1016/j.fcr.2010.04.010 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

