Effect of Reaction Media on Photosensitized [2+2]-Cycloaddition of Cinnamates
- PMID: 32499991
- PMCID: PMC7266492
- DOI: 10.1002/open.202000092
Effect of Reaction Media on Photosensitized [2+2]-Cycloaddition of Cinnamates
Abstract
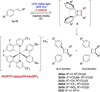
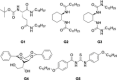
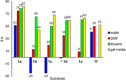
The outcome of photosensitized [2+2]-cycloaddition reactions of various cinnamates has been compared in different reaction media, including homogeneous organic solutions under inert conditions, degassed water, and aerated physical gels. The reactions were performed under LED blue light (λmax=455 nm) irradiation and [Ir{dF(CF3)ppy}2(dtb-bpy)]PF6 (1.0 mol%) as photocatalyst. The processes were optimized taking into consideration solvent, gelator, and substrate. Comparative kinetics analyses, as well as the effect of the reaction media on the diastereoselectivity of the process, were evaluated during this investigation. In a number of cases, carrying out the reaction in a less polar solvent, like toluene or highly polar solvent, like water had a tremendous impact on the diastereoselectivity of the process, pointing towards an effect on the stabilization of the putative diradical intermediate in this medium. Moreover, while for reactions run in homogeneous solution oxygen needs to be excluded, no erosion in yield is observed when the photoadditions were run in aerated gel media.
Keywords: [2+2]-cycloadditions; diastereoselectivity; diradicals; photocatalysis; photosensitizers; supramolecular gels.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Semiconductor Photocatalysis for Chemoselective Radical Coupling Reactions.Acc Chem Res. 2017 Apr 18;50(4):1002-1010. doi: 10.1021/acs.accounts.7b00023. Epub 2017 Apr 5. Acc Chem Res. 2017. PMID: 28378591
-
PCET-Based Ligand Limits Charge Recombination with an Ir(III) Photoredox Catalyst.J Am Chem Soc. 2021 Aug 25;143(33):13034-13043. doi: 10.1021/jacs.1c01701. Epub 2021 Aug 11. J Am Chem Soc. 2021. PMID: 34378919
-
Metal- and Oxidant-Free Photoinduced Aromatic Trifluoromethylation Performed in Aerated Gel Media: Determining the Effects on Yield and Selectivity.Molecules. 2018 Dec 21;24(1):29. doi: 10.3390/molecules24010029. Molecules. 2018. PMID: 30577645 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Rejuvenation of dearomative cycloaddition reactions via visible light energy transfer catalysis.Chem Sci. 2023 Oct 13;14(43):12004-12025. doi: 10.1039/d3sc04421a. eCollection 2023 Nov 8. Chem Sci. 2023. PMID: 37969572 Free PMC article. Review.
Cited by
-
Cage-confined photocatalysis for wide-scope unusually selective [2 + 2] cycloaddition through visible-light triplet sensitization.Nat Commun. 2020 Sep 16;11(1):4675. doi: 10.1038/s41467-020-18487-5. Nat Commun. 2020. PMID: 32938933 Free PMC article.
-
Studies on the Oxidation of Aromatic Amines Catalyzed by Trametes versicolor Laccase.Int J Mol Sci. 2023 Feb 9;24(4):3524. doi: 10.3390/ijms24043524. Int J Mol Sci. 2023. PMID: 36834934 Free PMC article.
References
-
- N. J. Turro, V. Ramamurthy, J. C. Scaiano, Modern molecular photochemistry of organic molecules, University Science Books, 2009.
-
- Matharu A. S., Ramanujam P. S. in Photochromic polymers for optical data storage: azobenzenes and photodimers, in Photochemistry and Photophysics of Polymer Materials (Eds.: M. S. Allen), John Wiley & Sons, Ltd, USA, 2010, pp. 209–234.
-
- Lohse B., Vestberg R., Ivanov M. T., Hvilsted S., Berg R. H., Hawker C. J., Ramanujam P. S., Chem. Mater. 2008, 20, 6715–6720.
-
- Iliopoulos K., Krupka O., Gindre D., Salle M., J. Am. Chem. Soc. 2010, 132, 14343–14345. - PubMed
-
- Ishikawa N., Furutani M., Arimitsu K., ACS Macro Lett. 2015, 4, 741–744. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

