Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products
- PMID: 32500220
- PMCID: PMC7829230
- DOI: 10.1007/s11239-020-02154-z
Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products
Erratum in
-
Correction to: Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products.J Thromb Thrombolysis. 2021 Jan;51(1):246. doi: 10.1007/s11239-020-02197-2. J Thromb Thrombolysis. 2021. PMID: 32578054 No abstract available.
-
Correction to: Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products.J Thromb Thrombolysis. 2021 Jan;51(1):247. doi: 10.1007/s11239-020-02311-4. Epub 2020 Oct 31. J Thromb Thrombolysis. 2021. PMID: 33128729 Free PMC article. No abstract available.
Abstract
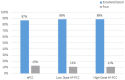
To analyze the efficacy and safety of activated prothrombin complex concentrates (aPCC) and four-factor prothrombin complex concentrates (4F-PCC) to prevent hematoma expansion in patients taking apixaban or rivaroxaban with intracranial hemorrhage (ICH). In this multicenter, retrospective study, sixty-seven ICH patients who received aPCC or 4F-PCC for known use of apixaban or rivaroxaban between February 2014 and September 2018 were included. The primary outcome was the percentage of patients who achieved excellent/good or poor hemostasis after administration of aPCC or 4F-PCC. Secondary outcomes included hospital mortality, thromboembolic events during admission, and transfusion requirements. Excellent/good hemostasis was achieved in 87% of aPCC patients, 89% of low-dose 4F-PCC [< 30 units per kilogram (kg)], and 89% of high-dose 4F-PCC (≥ 30 units per kg). There were no significant differences in excellent/good or poor hemostatic efficacy (p = 0.362). No differences were identified in transfusions 6 h prior (p = 0.087) or 12 h after (p = 0.178) the reversal agent. Mortality occurred in five patients, with no differences among the groups (p = 0.838). There were no inpatient thromboembolic events. Both aPCC and 4F-PCC appear safe and equally associated with hematoma stability in patients taking apixaban or rivaroxaban who present with ICH. Prospective studies are needed to identify a superior reversal agent when comparing andexanet alfa to hospital standard of care (4F-PCC or aPCC) and to further explore the optimal dosing strategy for patients with ICH associated with apixaban or rivaroxaban use.
Keywords: Direct oral anticoagulants; Factor xa inhibitors; Hematomas; Hemostatics; Intracranial hemorrhages; Prothrombin complex concentrates.
Conflict of interest statement
This manuscript has been read and approved for submission by all authors. There was no financial support provided for this manuscript. The authors declare they have no conflicts of interest.