Flexible motor sequence generation during stereotyped escape responses
- PMID: 32501216
- PMCID: PMC7338056
- DOI: 10.7554/eLife.56942
Flexible motor sequence generation during stereotyped escape responses
Abstract
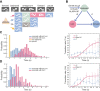
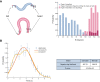
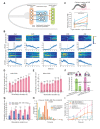
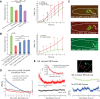
Complex animal behaviors arise from a flexible combination of stereotyped motor primitives. Here we use the escape responses of the nematode Caenorhabditis elegans to study how a nervous system dynamically explores the action space. The initiation of the escape responses is predictable: the animal moves away from a potential threat, a mechanical or thermal stimulus. But the motor sequence and the timing that follow are variable. We report that a feedforward excitation between neurons encoding distinct motor states underlies robust motor sequence generation, while mutual inhibition between these neurons controls the flexibility of timing in a motor sequence. Electrical synapses contribute to feedforward coupling whereas glutamatergic synapses contribute to inhibition. We conclude that C. elegans generates robust and flexible motor sequences by combining an excitatory coupling and a winner-take-all operation via mutual inhibition between motor modules.
Keywords: C. elegans; escape response; feedforward excitation; motor sequence generation; mutual inhibition; neuroscience; winner-take-all.
© 2020, Wang et al.
Conflict of interest statement
YW, XZ, QX, WH, JF, JH, TX, YX, MA, MZ, QW No competing interests declared
Figures






Comment in
-
Turning away from danger.Elife. 2020 Jul 21;9:e59910. doi: 10.7554/eLife.59910. Elife. 2020. PMID: 32691732 Free PMC article.
References
-
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge University Press; 1991.
-
- Ahamed T, Costa AC, Stephens GJ. Capturing the continuous complexity of behavior in C. elegans. arXiv. 2019 https://arxiv.org/abs/1911.10559
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

