Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease
- PMID: 32501293
- PMCID: PMC7406242
- DOI: 10.1172/jci.insight.139834
Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease
Abstract
Background: Identifying immune correlates of COVID-19 disease severity is an urgent need for clinical management, vaccine evaluation, and drug development. Here, we present a temporal analysis of key immune mediators, cytokines, and chemokines in blood of hospitalized COVID-19 patients from serial sampling and follow-up over 4 weeks.
Methods: A total of 71 patients with laboratory-confirmed COVID-19 admitted to Beijing You'an Hospital in China with either mild (53 patients) or severe (18 patients) disease were enrolled with 18 healthy volunteers. We measured 34 immune mediators, cytokines, and chemokines in peripheral blood every 4-7 days over 1 month per patient using a bioplex multiplex immunoassay.
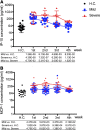
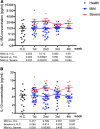
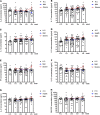
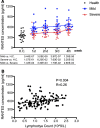
Results: We found that the chemokine RANTES (CCL5) was significantly elevated, from an early stage of the infection, in patients with mild but not severe disease. We also found that early production of inhibitory mediators including IL-10 and IL-1RA were significantly associated with disease severity, and a combination of CCL5, IL-1 receptor antagonist (IL-1RA), and IL-10 at week 1 may predict patient outcomes. The majority of cytokines that are known to be associated with the cytokine storm in virus infections such as IL-6 and IFN-γ were only significantly elevated in the late stage of severe COVID-19 illness. TNF-α and GM-CSF showed no significant differences between severe and mild cases.
Conclusion: Together, our data suggest that early intervention to increase expression of CCL5 may prevent patients from developing severe illness. Our data also suggest that measurement of levels of CCL5, as well as IL-1RA and IL-10 in blood individually and in combination, might be useful prognostic biomarkers to guide treatment strategies.
Keywords: Adaptive immunity; COVID-19.
Conflict of interest statement
Figures

References
-
- [No authors listed]. Coronavirus disease (COVID-19) pandemic. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed June 11, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

