SARS-CoV-2 infection serology: a useful tool to overcome lockdown?
- PMID: 32501411
- PMCID: PMC7249039
- DOI: 10.1038/s41420-020-0275-2
SARS-CoV-2 infection serology: a useful tool to overcome lockdown?
Abstract
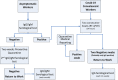
The outbreak of 2019 novel coronavirus disease (Covid-19) caused by SARS-CoV-2 has spread rapidly, inducing a progressive growth in infected patients number. Social isolation (lockdown) has been assessed to prevent and control virus diffusion, leading to a worldwide financial and political crisis. Currently, SARS-CoV-2 RNA detection in nasopharyngeal swab takes place by real-time PCR (RT-qPCR). However, molecular tests can give some false-negative results. In this context, serological assays can be useful to detect IgG/IgM antibodies, to assess the degree of immunization, to trace the contacts, and to support the decision to re-admit people at work. A lot of serological diagnostic kits have been proposed on the market but validation studies have not been published for many of them. The aim of our work was to compare and to evaluate different assays analytical performances (two different immunochromatographic cards, an immunofluorescence chromatographic card, and a chemiluminescence-automated immunoassay) on 43 positive samples with RT-qPCR-confirmed SARS-CoV-2 infection and 40 negative control subjects. Our data display excellent IgG/IgM specificities for all the immunocromatographic card tests (100% IgG and 100% IgM) and for the chemiluminescence-automated assay (100% IgG and 94% IgM); IgG/IgM sensitivities are moderately lower for all methods, probably due to the assay viral antigen's nature and/or to the detection time of nasopharyngeal swab RT-qPCR, with respect to symptoms onset. Given that sensitivities (around 94% and 84% for IgG and IgM, respectively) implicate false-negative cases and given the lack of effective vaccines or treatments, the only currently available procedure to reduce SARS-CoV-2 transmission is to identify and isolate persons who are contagious. For this reason, we would like to submit a flowchart in which serological tests, integrated with nasopharyngeal swab RT-qPCR, are included to help social and work activities implementation after the pandemic acute phase and to overcome lockdown.
Keywords: Immunochemistry; Viral infection.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures


References
-
- Ciotti, M. et al. COVID-19 outbreak: an overview. Chemotherapy, 1–9. [published online ahead of print]. (2020).
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation Report-91.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (2020).
-
- World Health Organization. Novel coronavirus (COVID-19) situation. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd. Accessed 24 March 2020 (2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

