Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study
- PMID: 32501454
- PMCID: PMC7252085
- DOI: 10.1016/S2665-9913(20)30127-2
Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study
Abstract
Background: Mortality of patients with coronavirus disease 2019 (COVID-19), acute respiratory distress syndrome (ARDS), and systemic inflammation is high. In areas of pandemic outbreak, the number of patients can exceed maximum capacity of intensive care units (ICUs), and, thus, these individuals often receive non-invasive ventilation outside of the ICU. Effective treatments for this population are needed urgently. Anakinra is a recombinant interleukin-1 receptor antagonist that might be beneficial in this patient population.
Methods: We conducted a retrospective cohort study at the San Raffaele Hospital in Milan, Italy. We included consecutive patients (aged ≥18 years) with COVID-19, moderate-to-severe ARDS, and hyperinflammation (defined as serum C-reactive protein ≥100 mg/L, ferritin ≥900 ng/mL, or both) who were managed with non-invasive ventilation outside of the ICU and who received standard treatment of 200 mg hydroxychloroquine twice a day orally and 400 mg lopinavir with 100 mg ritonavir twice a day orally. We compared survival, mechanical ventilation-free survival, changes in C-reactive protein, respiratory function, and clinical status in a cohort of patients who received additional treatment with anakinra (either 5 mg/kg twice a day intravenously [high dose] or 100 mg twice a day subcutaneously [low dose]) with a retrospective cohort of patients who did not receive anakinra (referred to as the standard treatment group). All outcomes were assessed at 21 days. This study is part of the COVID-19 Biobank study, which is registered with ClinicalTrials.gov, NCT04318366.
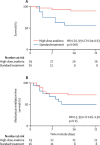
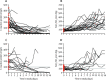
Findings: Between March 17 and March 27, 2020, 29 patients received high-dose intravenous anakinra, non-invasive ventilation, and standard treatment. Between March 10 and March 17, 2020, 16 patients received non-invasive ventilation and standard treatment only and comprised the comparison group for this study. A further seven patients received low-dose subcutaneous anakinra in addition to non-invasive ventilation and standard treatment; however, anakinra treatment was interrupted after 7 days because of a paucity of effects on serum C-reactive protein and clinical status. At 21 days, treatment with high-dose anakinra was associated with reductions in serum C-reactive protein and progressive improvements in respiratory function in 21 (72%) of 29 patients; five (17%) patients were on mechanical ventilation and three (10%) died. In the standard treatment group, eight (50%) of 16 patients showed respiratory improvement at 21 days; one (6%) patient was on mechanical ventilation and seven (44%) died. At 21 days, survival was 90% in the high-dose anakinra group and 56% in the standard treatment group (p=0·009). Mechanical ventilation-free survival was 72% in the anakinra group versus 50% in the standard treatment group (p=0·15). Bacteraemia occurred in four (14%) of 29 patients receiving high-dose anakinra and two (13%) of 16 patients receiving standard treatment. Discontinuation of anakinra was not followed by inflammatory relapses.
Interpretation: In this retrospective cohort study of patients with COVID-19 and ARDS managed with non-invasive ventilation outside of the ICU, treatment with high-dose anakinra was safe and associated with clinical improvement in 72% of patients. Confirmation of efficacy will require controlled trials.
Funding: None.
© 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- WHO Coronavirus disease 2019 (COVID-19): situation report—100. April 29, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

