Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis
- PMID: 32501510
- PMCID: PMC7478723
- DOI: 10.1084/jem.20192152
Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis
Abstract
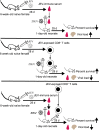
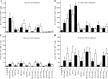
Cross-reactive anti-flaviviral immunity can influence the outcome of infections with heterologous flaviviruses. However, it is unclear how the interplay between cross-reactive antibodies and T cells tilts the balance toward pathogenesis versus protection during secondary Zika virus (ZIKV) and Japanese encephalitis virus (JEV) infections. We show that sera and IgG from JEV-vaccinated humans and JEV-inoculated mice cross-reacted with ZIKV, exacerbated lethal ZIKV infection upon transfer to mice, and promoted viral replication and mortality upon ZIKV infection of the neonates born to immune mothers. In contrast, transfer of CD8+ T cells from JEV-exposed mice was protective, reducing the viral burden and mortality of ZIKV-infected mice and abrogating the lethal effects of antibody-mediated enhancement of ZIKV infection in mice. Conversely, cross-reactive anti-ZIKV antibodies or CD8+ T cells displayed the same pathogenic or protective effects upon JEV infection, with the exception that maternally acquired anti-ZIKV antibodies had no effect on JEV infection of the neonates. These results provide clues for developing safe anti-JEV/ZIKV vaccines.
© 2020 Ningbo University.
Conflict of interest statement
Disclosures: S. Shresta reported a patent to "Flavivirus peptide sequences, epitopes, and methods and uses thereof," filed October 3, 2019, application no. 62/910,337 pending "La Jolla Institute." No other disclosures were reported.
Figures












Similar articles
-
Japanese encephalitis virus E protein domain III immunization mediates cross-protection against Zika virus in mice via antibodies and CD8+T cells.Virus Res. 2024 Jul;345:199376. doi: 10.1016/j.virusres.2024.199376. Epub 2024 Apr 20. Virus Res. 2024. PMID: 38643856 Free PMC article.
-
The effects of Japanese encephalitis virus antibodies on Zika virus infection.Med Microbiol Immunol. 2020 Apr;209(2):177-188. doi: 10.1007/s00430-020-00658-2. Epub 2020 Feb 20. Med Microbiol Immunol. 2020. PMID: 32078028
-
Japanese Encephalitis Virus Vaccination Elicits Cross-Reactive HLA-Class I-Restricted CD8 T Cell Response Against Zika Virus Infection.Front Immunol. 2020 Sep 25;11:577546. doi: 10.3389/fimmu.2020.577546. eCollection 2020. Front Immunol. 2020. PMID: 33101303 Free PMC article.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
-
The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis.PLoS Pathog. 2019 Apr 18;15(4):e1007640. doi: 10.1371/journal.ppat.1007640. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30998804 Free PMC article. Review.
Cited by
-
Protection against dengue virus requires a sustained balance of antibody and T cell responses.Curr Opin Virol. 2020 Aug;43:22-27. doi: 10.1016/j.coviro.2020.07.018. Epub 2020 Aug 13. Curr Opin Virol. 2020. PMID: 32798886 Free PMC article. Review.
-
Zika virus T-cell based 704/DNA vaccine promotes protection from Zika virus infection in the absence of neutralizing antibodies.PLoS Negl Trop Dis. 2024 Oct 17;18(10):e0012601. doi: 10.1371/journal.pntd.0012601. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39418312 Free PMC article.
-
Antibodies from dengue patients with prior exposure to Japanese encephalitis virus are broadly neutralizing against Zika virus.Commun Biol. 2024 Jan 24;7(1):15. doi: 10.1038/s42003-023-05661-w. Commun Biol. 2024. PMID: 38267569 Free PMC article.
-
Efficacy of genotype-matched vaccine against re-emerging genotype V Japanese encephalitis virus.Emerg Microbes Infect. 2024 Dec;13(1):2343910. doi: 10.1080/22221751.2024.2343910. Epub 2024 Apr 29. Emerg Microbes Infect. 2024. PMID: 38618740 Free PMC article.
-
A Zika virus mutation enhances transmission potential and confers escape from protective dengue virus immunity.Cell Rep. 2022 Apr 12;39(2):110655. doi: 10.1016/j.celrep.2022.110655. Cell Rep. 2022. PMID: 35417697 Free PMC article.
References
-
- Abbink P., Larocca R.A., De La Barrera R.A., Bricault C.A., Moseley E.T., Boyd M., Kirilova M., Li Z., Ng’ang’a D., Nanayakkara O., et al. . 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 353:1129–1132. 10.1126/science.aah6157 - DOI - PMC - PubMed
-
- Anderson K.B., Gibbons R.V., Thomas S.J., Rothman A.L., Nisalak A., Berkelman R.L., Libraty D.H., and Endy T.P.. 2011. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl. Trop. Dis. 5 e1311 10.1371/journal.pntd.0001311 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

