Noninfectious Granulomatous Diseases of the Chest
- PMID: 32501738
- PMCID: PMC7337224
- DOI: 10.1148/rg.2020190180
Noninfectious Granulomatous Diseases of the Chest
Abstract
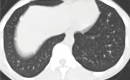
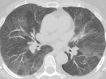
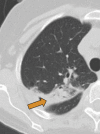
Granulomas are pathologically defined as focal aggregations of activated macrophages, Langerhans cells, and lymphocytes. Granulomas form in the lungs when the immune system barricades the substances it perceives as foreign but is unable to remove. Granulomas manifest with numerous imaging appearances in thoracic radiology, and their presence is a nonspecific finding. Granulomatous lung diseases comprise multiple entities with variable clinical manifestations and outcomes. Their imaging findings are rarely specific and can mimic malignancies, often triggering an extensive diagnostic workup. Radiologists must be familiar with the clinical manifestations and imaging findings of these entities to generate appropriate differential diagnoses. This review describes the imaging manifestations of various noninfectious, necrotizing, and nonnecrotizing granulomatous diseases that primarily affect the thorax. ©RSNA, 2020.
Figures







































References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

