Integrated Biophysical Modeling and Image Analysis: Application to Neuro-Oncology
- PMID: 32501772
- PMCID: PMC7520881
- DOI: 10.1146/annurev-bioeng-062117-121105
Integrated Biophysical Modeling and Image Analysis: Application to Neuro-Oncology
Abstract
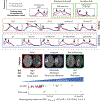
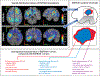
Central nervous system (CNS) tumors come with vastly heterogeneous histologic, molecular, and radiographic landscapes, rendering their precise characterization challenging. The rapidly growing fields of biophysical modeling and radiomics have shown promise in better characterizing the molecular, spatial, and temporal heterogeneity of tumors. Integrative analysis of CNS tumors, including clinically acquired multi-parametric magnetic resonance imaging (mpMRI) and the inverse problem of calibrating biophysical models to mpMRI data, assists in identifying macroscopic quantifiable tumor patterns of invasion and proliferation, potentially leading to improved (a) detection/segmentation of tumor subregions and (b) computer-aided diagnostic/prognostic/predictive modeling. This article presents a summary of (a) biophysical growth modeling and simulation,(b) inverse problems for model calibration, (c) these models' integration with imaging workflows, and (d) their application to clinically relevant studies. We anticipate that such quantitative integrative analysis may even be beneficial in a future revision of the World Health Organization (WHO) classification for CNS tumors, ultimately improving patient survival prospects.
Keywords: biophysical modeling; glioblastoma; image analysis; model calibration; multi-parametric imaging; radiomics; tumor growth.
Figures




References
-
- Collins VP. 1998. Gliomas. Cancer Surv. 32:37–51 - PubMed
-
- Mang A, Gholami A, Davatizkos C, Biros G. 2018. PDE-constrained optimization in medical image analysis. Optim. Eng 19(3):765–812
-
- Kleihues P, Burger PC, Scheithauer BW. 1993. The new WHO classification of brain tumours. Brain Pathol. 3:255–68 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

